Cannabis derived food products – what’s the current state of play?
Posted: February 25, 2019 | Author: Karin Verzijden | Filed under: Authors, cannabidiol, cannabis, Enforcement, Food, Health claims, novel food | Comments Off on Cannabis derived food products – what’s the current state of play? Recently, CBD food products were qualified as Novel Foods requiring a market authorization. The lively trade in these products therefore currently seems to be at risk. However, not all cannabis derived products are Novel Foods. What is the current state of play regarding these products and how is enforcement going to look like?
Recently, CBD food products were qualified as Novel Foods requiring a market authorization. The lively trade in these products therefore currently seems to be at risk. However, not all cannabis derived products are Novel Foods. What is the current state of play regarding these products and how is enforcement going to look like?
Current state of play re. cannabis derived products
In the European Union, the cultivation of Cannabis sativa L. varieties is permitted provided they are registered in the EU’s ‘Common Catalogue of Varieties of Agricultural Plant Species’ and the tetrahydrocannabinol (THC) content does not exceed 0.2 % weight per weight. The Common Catalogue is embodied in the EC Plant Variety database, which currently lists 68 species of Cannabis sativa. Some products derived from the Cannabis sativa plant or plant parts such as seeds, seed oil, hemp seed flour and defatted hemp seed have a history of consumption in the EU and therefore, in principle, are not novel.
What’s new?
This is different for extracts derived from Cannabis sativa L. and derived products containing cannabinoids, such as cannabidiol (CBD). It follows from a recent clarification of the Novel Food Catalogue that these products are considered Novel Foods, as a history of consumption regarding these products has not been demonstrated. This applies to both the extracts themselves and any products to which they are added as an ingredient. If for instance CBD is added to hemp seed oil, the product can no longer be marketed just like that and requires market authorization. The status of Novel Food also applies to extracts of other plants containing cannabinoids and to synthetically obtained cannabinoids.
How does the market of CBD food products currently look like?
Currently, the market in CBD food products is flourishing. A variety of CBD nutraceutical products is being offered for sale, such as HempFlax CBD, CBD oil, but also CBD-infused tea, honey or sweets. Although there is no hard-scientific evidence, many health benefits are connected to CBD food products, such as stress reduction, good night rest and providing energy and increasing resistance. Contrary to products containing THC (tetrahydrocannabinol), which is also extracted from cannabis, you do not get high on CBD food products, as this is not a psychoactive substance.
Medicinal use of cannabis
The use of cannabis derived CBD in food is not to be confused with medicinal use of cannabis. In most cases of medicinally applied cannabis, the active ingredient is THC, not just CBD, or a combination of THC and CBD. Although medicinally applied cannabis does not play a role in the cure of diseases, scientific publications show it can alleviate suffering from diseases, for instance nausea, decreased appetite, slimming or weakening due to cancer.
Consequences for business of the change in legal framework
Due to the qualification of CBD food products as Novel Foods, the lively trade in these products is currently at risk. Any Novel Food has to obtain a market authorization in order to get market access. CBD food products currently marketed may face enforcement measures, unless they can benefit from the transition regime laid down in the Novel Foods Regulation. According to this transition regime, any product that did not fall within the scope of the former Novel Foods Regulation, was lawfully marketed prior to 1 January 2018 and for which an application for market authorization is filed before 2 January 2020, can continue to be marketed until an authorization decision has been taken. While this transition period is in principle drafted for Novel Foods that fall into one of the new novel food categories under the new Novel Foods Regulation, it is in the spirit of the transition regime to also include the CBD scenario.
Pending CBD-application and expected EFSA opinion
Currently, one application for the authorization of a CBD food supplement is pending. The application was made by the company Cannabis Pharma from the Czech Republic and is based on publicly available safety and toxicological information and toxicity reviews. More in particular, the scientific data has been gathered from acute and long-term toxicity studies in animals and tolerance studies in humans. The data package submitted aims to support the safety of the use of CBD in food supplements for adults with a daily intake of up to 130 mg or 1.86 mg/kg body weight. It is reported by various sources that an EFSA opinion is awaited this March (see here and here).
Any benefits for the CBD market of a positive EFSA opinion?
Contrary to the situation under the former Novel Foods Regulation, the authorizations granted under the current Regulation have a generic nature. This means that any other company meeting the conditions of use stated in the authorization, would be at liberty to market CBD food supplements as well. The pending application made by Cannabis Pharma is therefore followed with great interest by the CBD market. As they do not seem to rely on data protection, a granted authorization would pave the way for other food supplement companies. It is not certain if this will happen still this year. If and when EFSA grants a positive opinion this March, the European Commission still has 7 months to submit an implementing act to the PAFF Committee. Upon a positive opinion of the PAFF committee, such implementing act could be quickly adopted. If the PAFF Committee has no opinion or a negative opinion, 1 or 2 months should be added to the procedure as a minimum.
Ireland: some CBD food products can be marketed
Meanwhile, there is some guidance available at Member State level. The Irish Food Safety Authority notes that recently a large number of CBD food products entered the market, typically marketed as food supplements in liquid or capsule form. Depending on the manufacturing process applied, the trade in CBD oil is not prohibited, as this oil naturally contains low levels of CBD, which is considered a non-psychoactive compound. This applies to CDB oil obtained by cold-pressing the hemp seeds. If and when the oil is obtained by supercritical CO2 extraction, then a Novel Food authorization is mandatory.
Denmark: available guidance not crystal clear
According to the Danish Ministry of Environment and Food, a number of Cannabis-derived products are not considered Novel Food, notably hemp seeds, seed flour, protein powder from seeds and seed oil from the Cannabis sativa L. varieties listed in the EC Plant Variety Database that are free from or contain low levels of THC. If these products contain CBD, the regulatory status is not exactly clear. According to the guidance of the Danish Food Ministry, the current status is that pure cannabidiol as well as hemp products with high (concentrated) levels of CBD or other cannabinoids are covered by the Novel Foods Regulation. It is not explained what is understood by “high levels of CBD”, but on the other hand an absolute prohibition to market these products in Denmark does not seem to apply.
Absolute prohibitions: Belgium and Austria
Other Member States seem to be stricter than Ireland or Denmark. For instance, the Austrian Health Ministry has made it perfectly clear that food products containing any type of cannabinoid extract without a Novel Food authorization are prohibited to be put on the market. In Belgium, the Federal Agency on Safety in the Food Chain has clarified that the production and marketing of food products based on cannabis is prohibited. The rationale is that the plant Cannabis sativa is mentioned in an annex to a national Decree listing dangerous plants that cannot be used for food production. The prohibition primarily seems to target the potentially dangerous substance of THC and allows derogations on a case-by-case basis, but not regarding food products containing CBD. These are considered Novel Foods requiring a market authorization.
Enforcement directed against (medical) claims in the Netherlands
Until CBD was declared a Novel Food, the trade in CBD food products was not prohibited in the Netherlands. Contrary to the substance TCH, the substance CBD is not mentioned in the Dutch Opium Act, listing prohibited substances having a psycho active effect. This does not mean that the trade in CBD food products was allowed just like that. In practice, enforcement in the Netherlands has been directed against the use of any unauthorized medical claims. A medical claim is any information according to which a food product could have a therapeutic or prophylactic effect. When using such a claim, one comes into the realm of the Medicinal Product Act, according to which it is prohibited to market and advertise any medicinal product without a market authorization. The Dutch Food Safety Authority announced fines up to
€ 10.000 regarding the sale of CBD food products in several cases (see here and here). Any food business operator that is serious about his business in CBD food products will therefore not only check the applicability of the Novel Foods Regulation to his products, but also carefully draft his advertisement for this type of product.
Conclusion
The production and marketing of food products derived from Cannabis sativa L. in the EU has been considerably restricted since CDB food products were recently declared to be Novel Foods. However, not all cannabis-derived food products require market authorization. Pending the evaluation of the Novel Food application filed for a CBD food supplement by the Czech company Cannabis Pharma, it is worthwhile for other CBD food products to verify whether they can benefit from the so-called transition regime embodied in the Novel Foods Regulation. Due to differences between legislation in the Member States, this may differ from country to country. Also, it is important to carefully position your CBD food product, in order to avoid any medical claims.
The author ackowledges Jasmin Buijs, paralegal at Axon, and Max Luijkx, intern at Axon, for their valuable input.
How do we get cellular ag products to the market?
Posted: September 25, 2018 | Author: Karin Verzijden | Filed under: Authors, Food, Information, novel food | Comments Off on How do we get cellular ag products to the market? “The FDA knows just how vital it is to ensure the safety of our nation’s food supply and the critical role science-based, modern regulatory frameworks are to fostering innovation. Recent advances in animal cell cultured food products present many important and timely technical and regulatory considerations for the FDA and our partners at USDA,” said Commissioner Gottlieb. “We look forward to the opportunity to hold a meeting with our USDA colleagues as part of an open public dialogue regarding these products.”
“The FDA knows just how vital it is to ensure the safety of our nation’s food supply and the critical role science-based, modern regulatory frameworks are to fostering innovation. Recent advances in animal cell cultured food products present many important and timely technical and regulatory considerations for the FDA and our partners at USDA,” said Commissioner Gottlieb. “We look forward to the opportunity to hold a meeting with our USDA colleagues as part of an open public dialogue regarding these products.”
Harvard Law School
This is a new quote taken from the website of the FDA, regarding a joint FDA – USDA meeting on animal cell culture technology on 23 and 24 October 2018 in Washington. Securing regulatory clearance is pivotal for market access of this type of products, which used to be often referred to as “clean meat,” “cell-based meat,” or “lab-grown meat,” depending on whom you would ask. Since the Good Food Conference early September, there seems to be consensus to retain the term “cell-based meat”. To date, there is however no consensus on the appropriate US regulatory framework for these products, which is why this new meeting has been set. In particular, it is not clear which federal regulatory agency – the FDA or USDA or both – has jurisdiction over these products with respect to labeling, safety and inspections, or whether these products will meet regulatory definitions relating to “meat” and “poultry.” Harvard Law School, in particular its representatives from the Food Law and Animal Law groups, recognizes the potential benefits of clean meat and other cellular agriculture products. Therefore, on 9 and 10 August 2018, the school organized the Clean Meat Regulatory Roundtable in order to address the regulatory concerns surrounding this new industry. Further to an earlier post on this subject, this is take #2 on the Harvard initiative, reporting a selection of topics discussed at that table. Also a comparison with the EU regulatory framework shall be made where relevant.
Participants Regulatory Round Table and Ruled of Play
In order to let the discussion benefit from as many perspectives as possible, representatives from academia, the industry and from interest groups participated in Harvard’s Clean Meat Regulatory Roundtable. Obviously, the meeting included a number of representatives from Harvard Law School. Furthermore, the nonprofit organizations Good Food Instituteand the Animal League Defense Fund were present. The participating companies were Mosa Meat and Fork & Goode (both working on clean meat), as well as Blue Nalu (working on clean fish). From the investor side, Stray Dog Capital was represented and from the industry DuPont provided its input. Finally, a number of US lawyers participated, including Deepti Kulkarni from Sidley Austin LLP. I myself shared my insights on this topic from an EU perspective. To encourage openness and the sharing of information, all participants agreed to a Chatham House Rules + regime, meaning that the topics discussed during the meeting could be reported, but the participants were not at liberty to identify, either explicitly or implicitly, the identity of the speaker or his or her affiliation.
Cooperation on optimal regulatory pathway?
Amongst the participants, there was a common concern that if one party rushes unprepared to the market, ignoring the appropriate regulatory pathways, that could poison the well for the entire cellular agricultural industry. It was therefore discussed to what extent the cellular agriculture industry could cooperate regulatory-wise. It became clear that even though clean meat products share certain characteristics, finished products could be quite different in terms of formulation, composition, or other product characteristics. Nonetheless, general principles for assessing safety and product identity could be established under existing regulatory authorities. Such principles could for instance involve considerations relating to substances used in the production of clean meat, including scaffolds, growth factors, and cell culture medium or potential variations in the production process. In addition, it was discussed whether common interests could be effectively promoted through a trade association or industry group. The participants discussed various options, including setting up a dedicated association and connecting with existing organizations such as BIO.
Agency Jurisdiction
Contrary to the situation in the EU, where the European Commission is the one stop shop for obtaining a Novel Foods authorization, in the US foods can be subject to regulatory oversight by multiple federal and state agencies. It is has not yet been decided which agency has jurisdiction over these products, or whether the products are subject to dual oversight. In general, USDA regulates meat and poultry, including the inspection of establishments that slaughter such animals or otherwise process meat and poultry products. FDA generally regulates all other food, including fish and certain other meat and poultry products, such as bison, rabbits, and wild turkeys and ducks. In addition, FDA regulates new ingredients (as “food additives”) used in the production of foods under its jurisdiction, as well as new ingredients used in meat and poultry products that otherwise would be subject to USDA oversight.
Continuous inspections?
Meat and poultry products subject to USDA jurisdiction generally require “continuous” or daily inspection, depending upon the nature and frequency of operations. Because the production of clean meat does not involve slaughtering animals and such products would not be derived from slaughtered animals, there are open questions regarding the applicability of USDA’s inspection regime. This is even more so when clean meat products are not “harvested” daily, but on a batch-based basis. Arguments, nonetheless, can be made in favor of USDA jurisdiction with respect to “processing” inspections and other in-market activities, as well as product labeling. Nevertheless, at the Roundtable, there seemed to be a slight preference for FDA jurisdiction both pre-market and in-market.
Traditional meat industry’s concern of level playing field
The views of the traditional meat industry are somewhat fractured with respect to the clean meat industry. Some have taken considerable stakes in clean meat ventures (consider the investments of both Tyson and Cargill in Memphis Meats), particularly industry segments more closely involved with raising livestock are less supportive. For example, the US Cattleman’s Association submitted a petition to the USDA in February this year, asking it to establish labeling requirements that would prohibit clean meat from using terms like “meat” or “beef” in product labeling. Most trade groups representing the traditional meat industry have called for a “level playing field” where clean meat products would be subject to some level of USDA inspection. In general, all participants at the Harvard Panel agreed that a discussion with the traditional meat industry on how clean meat should be regulated would be critical. Notably, clean meat producer Memphis Meats and the North American Meat Institute, a trade group that represents the largest meat producers in the U.S. recently issued a joint letter to the White House outlining a regulatory regime under both FDA and USDA, and calling for a combined meeting involving the White House, USDA, FDA, and representatives of the traditional meat and clean meat industries.
Pre-market evaluation
Even if the FDA were to have some level of jurisdiction over clean meat products, it is still not completely clear what data would need to be submitted to demonstrate safety. One of the participants opined that, unlike a food additive approval, neither a GRAS determination, nor an FDA consultation (see here for an example of a consultation procedure on plant based products) would result in an affirmative regulatory approval. Some were of the opinion that a regulatory opinion short of approval would not benefit the clean meat industry, particularly in the eyes of the public. By contrast, others particularly those familiar with the GRAS process and the requisite scientific information needed to demonstrate safety disagreed with the position that a GRAS determination would not be rigorous or otherwise appropriate. The participants then discussed that a potential hybrid model that followed the GRAS approach, but also involved a third-party safety opinion could be an option. As to the required data, it was discussed what would be the “ingredient” that would be assessed by the competent government agency. The substances used in the culture of clean meat products most likely are of relevance, even if they may qualify as mere processing aids that normally only remain as residues in the final product without technical function. One participant mentioned such substances could easily be tested in separate toxicology studies, to which reference could be made during the pre-market evaluation (US) or in the application for a Novel Foods authorization (EU).
EU market entry of Novel Foods
Compared to the US, the regulatory pathway for clean meat products in the European Union is relatively clear. Under the new Novel Foods Regulation (effective as per 1 January 2018), an application for an authorization of a Novel Food should be made with the European Commission, who will subsequently distribute this to all EU Member States. The application should in the first place contain a detailed description of the product for which an authorization is sought, as well as of its production process. Furthermore, a proposal for the purported conditions of use should be handed in and a labelling proposal that does not mislead the consumer. Last but not least, the applicant should provide scientific evidence, demonstrating the purported Novel Food does not pose a safety risk. For this purpose, tox studies that comply with Good Laboratory Practices are mandatory, as is an evaluation of the total safety strategy. This should be based on proposed uses and likely exposure, with justification to include or exclude certain studies in order to prevent cherry picking. Upon receipt of the Novel Food application, it is anticipated that the Commission will request a safety opinion from EFSA, who will evaluate, amongst other things, if the Novel Food concerned is as safe as food from a comparable food category already placed on the EU market. The EFSA evaluation should not exceed a 9 months term. Within 7 months after receipt of a positive safety opinion, the Commission should publish its implementing act, which will result in the inclusion of the approved Novel Food in the Union List. The single open end in this procedure is the term for response for the Member States, which in the former Novel Food Regulation used to be 60 days. Surprisingly enough, this term is not mentioned in the new Novel Food Regulation that applies as of 1 January this year. However, there are no reasons to believe this should be any different under the current Novel Foods Regulation.
In-Market Safety
In order to inspire consumer confidence in clean meat products, the participants discussed how to best ensure the products’ short and long-term safety, particularly against the backdrop of public fear and aversion to genetically modified foods. Despite the assurance that FDA provided regarding the safety of these foods, many consumers remain fearful or otherwise suspicious of such foods. The participants agreed that steps should be taken to avoid a similar unwarranted aversion to clean meat products, including transparency initiatives and consumer education. In this framework, it was suggested that the clean meat industry could pro-actively develop its own HACCP program, provided that the industry could reach agreement on what would be the best way to identify the hazards and applicable critical control points. To this respect, it is relevant that both FDA and USDA have HACCP regulations and have identified hazards of chemical, biological and physical nature that might be applicable to this new sector.
Labeling, marketing, product identity
Vivid discussions took place regarding whether or not clean meat or fish products could be called “meat” or “fish” respectively. Whereas some argued: “Meat is what it is, so meat it should be called”, others considered the actual name less important. Most likely, we will not see the plain term “meat” on product packaging, but rather “ground beef,” “meatballs,” or “chicken tenders”. Some participants cautioned that, in order for products to be labeled with such product-specified terms, they generally would have to meet the general definitions for “meat” or “poultry,” unless the labeling adequately described or qualified the product. For product placement, it is of relevance whether the clean meat products belong to the “meat department” or somewhere else, though several participants clarified that such placement is decided by agreements with retailers, rather than by regulatory oversight. All participants agreed that a so-called qualifier that would explain the exact nature of the product, could benefit the industry. Such qualifier should be a neutral term, explaining concisely how clean meat products differ positively from traditional meat products, without being pejorative vis-à-vis said traditional industry. This is easier said than done and the participants so far did not reach a common view at this point. Notably, in their joint letter to the White House, Memphis Meats and the North American Meat Institute, propose use of the term “cell-based” meat or poultry to describe products that are the result of animal cell culture. The echo of this letter was heard at the Good Food Conference, as reported above.
Information requirements in the EU
Looking at the EU framework, it is questionable whether the designation “meat” can be used for clean meat products. As an argument in favor thereof, it could be mentioned such use would make it immediately apparent that these products equal traditional meat in terms of composition. Arguments countering the use of “meat” are based on the EU Hygiene Regulation. When using a grammatical approach, it should be observed that “meat” is defined as “edible parts of [a number of defined]animals” and one could wonder if cells qualify as such. When using a functional interpretation, it can be noted that hygiene requirements applicable to meat mainly relate to slaughtering, whereas clean meat obviously is not subject to slaughter. Another argument countering the use of “meat” for clean meat products is derived from the ECJ’s TofuTown case, related to veggie cheese and soy yoghurt. In legal terms, this decision answered the question whether it was permitted to use regulated product names for new product types. The answer was a clear “no”. It is anticipated that the traditional meat industry will rely on this case to counter the use of the name “meat” for clean meat product, at least without a qualifier that will prevent any misleading of the consumer. By way of background, it is helpful to remember that under EU labeling laws, it is mandatory to designate a food product by its legal name. In the absence thereof, a descriptive name can be used or alternatively, a customary name.
Summary
Clean meat and clean fish represent an emerging sector, with the promise of revolutionary innovative products. Public perception of these products, as well as trust in the safety thereof, will be of utmost importance for market success. Reliable and effective regulatory procedures as a basis for market access will therefore be pivotal. In the US, the regulatory framework applicable to clean meat products is far from clear. Firstly, it is yet to be decided which government agency has jurisdiction over these products or whether both FDA and USDA share oversight. Secondly, there are open questions regarding the appropriate regulatory pathway and in-market inspection regime. In the European Union, the regulatory pathway for clean meat products is relatively clear. Under the new EU Novel Foods Regulation, these products qualify as Novel Foods and require a market authorization from the European Commission. The Regulation as well as various EFSA guidance documents detail at length what information should be contained in a Novel Foods application. In an optimal situation, the authorization procedure could be finalized in 18 months. In both the US and the EU however, the exact designation of these products (“meat” or not?) requires further thought. On the one hand, this will require interpretation of legal product definitions and case-law and on the other hand, the interests of the traditional meat and fish sector should be taken into account.
The Dutch National Probiotic Guide: an innovative alternative for health claims on beneficial bacteria
Posted: August 24, 2018 | Author: Jasmin Buijs | Filed under: Authors, Food, Food Supplements, Health claims, Information | Comments Off on The Dutch National Probiotic Guide: an innovative alternative for health claims on beneficial bacteria Probiotics are known as “beneficial bacteria” that can be found in, amongst others, dairy products and food supplements. They are defined by the joint FAO/WHO expert consultation on probiotics as “live microorganisms that, when administrated in adequate amounts, confer a health benefit on the host”. Since the reference to probiotics implies a health benefit, it comes as no surprise that the statement “contains probiotics” in a commercial communication about a food product constitutes a health claim under the Claims Regulation. Moreover, “contains probiotics”, or “prebiotics”, is explicitly taken as an example of a health claim in the guidance on the implementation of Regulation 1924/2006 of the European Commission’s Standing Committee on the Food Chain and Animal Health. At this moment, no health claims for probiotics have been approved by the European Commission. The Dutch Research institute TNO and the world’s first microbe museum Micropia, located in Amsterdam, are nevertheless convinced of the health benefits of probiotics, in particular to protect against antibiotic-associated diarrhea (AAD). At the beginning of this month, they launched a so-called National Guide on clinically proven probiotics for use during antibiotic treatment in the scientific journal BMC Gastroenterology
Probiotics are known as “beneficial bacteria” that can be found in, amongst others, dairy products and food supplements. They are defined by the joint FAO/WHO expert consultation on probiotics as “live microorganisms that, when administrated in adequate amounts, confer a health benefit on the host”. Since the reference to probiotics implies a health benefit, it comes as no surprise that the statement “contains probiotics” in a commercial communication about a food product constitutes a health claim under the Claims Regulation. Moreover, “contains probiotics”, or “prebiotics”, is explicitly taken as an example of a health claim in the guidance on the implementation of Regulation 1924/2006 of the European Commission’s Standing Committee on the Food Chain and Animal Health. At this moment, no health claims for probiotics have been approved by the European Commission. The Dutch Research institute TNO and the world’s first microbe museum Micropia, located in Amsterdam, are nevertheless convinced of the health benefits of probiotics, in particular to protect against antibiotic-associated diarrhea (AAD). At the beginning of this month, they launched a so-called National Guide on clinically proven probiotics for use during antibiotic treatment in the scientific journal BMC Gastroenterology
National Guide
The National Guide is presented as a tool for healthcare professionals, patients and other consumers to recommend or use the probiotic products listed as scientifically proven to prevent diarrhea caused by the use of antibiotics. While antibiotics fight bacterial pathogens, they also have a disruptive effect on the body’s own gut bacteria. One in four adults experiences diarrhea caused by ADD. The National Guide promotes probiotics for their function of protecting the gut flora from the disruptive effects of antibiotic treatment, fostering recovery and reducing the risk of recurring infections.
Science-based approach
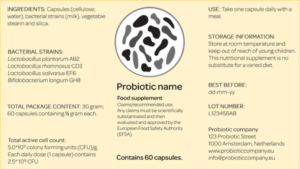
The research behind the Guide involves a literature study of clinical studies that are all based on randomized, double-blind and placebo-controlled trials. Moreover, all of the trials clearly define AAD and have a probiotic administration regime for a period no shorter than the antibiotic therapy. 32 of the 128 initially identified clinical studies were selected in line with the aforementioned criteria. After the selection and review process, available probiotic products on the Dutch market were listed to be subsequently matched with the formulations as proven effective in the selected clinical studies. Only eight probiotic dairy products and food supplements marketed in the Netherlands specified on their label the respective probiotic strain(s) and number of colony-forming units (CFUs) and could therefore be used in the research. The listed probiotic products were awarded with one (lowest) to three (highest) stars for their proven effect as demonstrated in at least one to three clinical studies. The strain Lactobacillus rhamnosus GG with a minimal daily dose of 2 × 109 CFU was found in at least three clinical studies and therefore awarded with a three-star recommendation. This strain was found in 2 products, both of which are food supplements. Several multi-strain formulations resulted in a one-star recommendation; 5 food supplements and 1 dairy product matched such a formulation. The multi-strain formulation Lactobacillus rhamnosus GG, Lactobacillus acidophilus LA-5 and Bifidobacterium lactis BB-12 was present in two clinical studies and therefore assigned with a two-star recommendation. However, none of the listed probiotic products found on the Dutch market contained this formulation.
 Plea for the labeling of probiotics
Plea for the labeling of probiotics
The research is not exhaustive as probiotic products other than the eight that were included in the study might also be effective. However, since this was not communicated on the label, they could not be included in the research. To overcome this gap, TNO and Micropia as the initiators of the National Guide call for the labeling of the probiotic strains and number of CFUs on all probiotic products EU-wide. This could also expand the potential of the Guide. At this moment, strain and CFU labeling of probiotic products is not legally mandatory under the Food Information for Consumer Regulation. The initiators also developed a special probiotic label to address this claimed deficiency. The label is based on the probiotic label used in the US as created by the International Scientific Association for Probiotics and Prebiotics (ISAPP). The labels are in line with the information that should be demonstrated on probiotic labels according to the FAO/WHO 2002 Working Group on Guidelines for the Evaluation of Probiotics in Food.
National Guide to circumvent limitations under the Claims Regulation?
The Claims Regulation applies to health (and nutrition) claims made in commercial communications of foods to end consumers. This may be in the labeling, presentation or advertising of the food. Besides information on or about the product itself, also general advertising and promotional campaigns such as those supported in whole or in part by public authorities fall within the scope of the Regulation. Moreover, since the Innova Vital case, we know that (science-based) communications made to healthcare professionals may also be regulated by the Claims regulation. The rationale thereof is that the healthcare professional can promote or recommend the food product at issue by passing the information on to the patient as end consumer. Only non-commercial communications, such as publications that are shared in a purely scientific context, are excluded from the Regulation.
It must be noted that the National Guide is, unlike health claims, not a commercial communication originating from food business operators. This does, however, not necessarily mean that food business operators are free to use the science-based Guide in their communication with (potential) consumers or even with healthcare professionals without any reservation. The Guide, which not only lists the probiotic formulations that are beneficial for the human gut flora, but even the names of products that contain those formulations, could turn commercial when referred to by a food business. Moreover, when shared in such a context, the claims made in the National Guide may even enter the medical domain due to the preventive function assigned to foods containing probiotics.
Conclusion
The history of probiotic health claim applications has shown that EFSA is not easily convinced of the evidence that is correspondingly provided. The National Guide is, however, not subject to approval from the European Commission, backed by a positive opinion from EFSA. The Guide’s publication in the peer-reviewed journal BMC Gastroenterology nevertheless contributes to the verification of its scientific substantiation. The Guide therefore appears as an innovative, science-based alternative for probiotic health claims. At the same time, food business operators should be careful in referring to the National Guide to not act beyond the borders of the Claims Regulation and to stay away from medical claims. As a very minimum however, it seems to be valuable work to be adopted by branch organizations or research exchange platforms, such as the International Probiotics Association.
Take # 1 on Harvard’s Clean Meat Regulatory Roundtable (9-10 August 2018)
Posted: August 16, 2018 | Author: Karin Verzijden | Filed under: Authors, novel food | Comments Off on Take # 1 on Harvard’s Clean Meat Regulatory Roundtable (9-10 August 2018) Cultured meat has been an increasingly hot topic since the first “clean meat” hamburger was introduced in London in 2013. The technology involves using cell culturing techniques to multiply a small amount of cells taken from an animal to produce foods that resemble traditional meat, poultry, and seafood. As commercial-scale production of cultured meat becomes foreseeable, regulatory agencies must determine how these products fit into their food compliance programs.”
Cultured meat has been an increasingly hot topic since the first “clean meat” hamburger was introduced in London in 2013. The technology involves using cell culturing techniques to multiply a small amount of cells taken from an animal to produce foods that resemble traditional meat, poultry, and seafood. As commercial-scale production of cultured meat becomes foreseeable, regulatory agencies must determine how these products fit into their food compliance programs.”
Harvard Law School
This is a quote taken from the website of the FDA, who organized a public meeting on 12 July 2018 to discuss safety-related data and information that the FDA is seeking on foods produced using animal cell culture techniques. Securing regulatory approval is pivotal for market access of this type of products. To date, there is no consensus how the US regulatory apparatus will classify Clean Meat products. It is neither clear which authority has jurisdiction over these products as to labeling, safety and inspections. Harvard Law School, in particular its representatives from the Food Law and Animal Law groups, is convinced of the potential benefits of Clean Meat and other cellular agriculture products. Therefore, on 9 and 10 August 2018, it organized the Clean Meat Regulatory Roundtable in order to address the regulatory concerns surrounding this new industry. A selection of topics discussed will be covered in two subsequent blogposts. The first one will cover some background information and a summary. The second one will provide an overview of the most important subjects discussed and a comparison with the EU regulatory framework where relevant.
Participants Regulatory Round Table and Ruled of Play
In order to let the discussion benefit from as many perspectives as possible, representatives from academia, the industry and from interest groups participated in Harvard’s Clean Meat Regulatory Round Table. Obviously, the meeting included a number of representatives from Harvard Law School. Furthermore, the nonprofit organizations Good Food Institute and the Animal League Defense Fund were present. The participating companies were Mosa Meat and Fork & Goode (both working on clean meat), as well as Blue Nalu (working on clean fish). From the investor side, Stray Dog Capital was represented and from the industry DuPont provided its input. Finally, a number of US lawyers were participating and I myself shared my insights from an EU perspective. All participants agreed to a Chattam House Rules + regime, meaning that the general flush of the conversation during the meeting can be reported, but the participant are not at liberty to identify, either explicitly or implicitly, what was the source of particular information.
Summary
Clean meat and clean fish represent an emerging sector, with the promise of revolutionary innovative products. Public perception of these products, as well as trust in the safety thereof, will be of utmost importance for market success. Reliable and effective regulatory procedures as a basis for market access will therefore be pivotal. In the US, the regulatory framework applicable to clean meat products is far from clear. Firstly, it is yet to be decided which government agency has jurisdiction over these products. Secondly, the regulatory pathway is still open, whereas the available procedures of do not provide an affirmative blessing from which the sector could benefit. In the European Union, the regulatory pathway for clean meat products is relatively clear. Under the new Novel Foods Regulation (effective as per 1 January 2018), these products qualify as Novel Foods and require a market authorization from the European Commission. The Regulation as well as various EFSA guidance documents detail at length what information should be contained in a Novel Foods application. In an optimal situation, the authorization procedure could be finalized in 18 months. In both the US and the EU however, the exact designation of these products (“meat” or not?) requires further thought. On the one hand, this will require interpretation of legal product definitions and case-law and on the other hand, the interests of the traditional meat and fish sector should be taken into account.
Dutch scoop: new Advisory Group on the Status of Borderline Products
Posted: July 16, 2018 | Author: Karin Verzijden | Filed under: Authors, Enforcement, Food, Health claims, Nutrition claims | Comments Off on Dutch scoop: new Advisory Group on the Status of Borderline Products Last week, the creation of the new Advisory Group on the Status of Borderline Products was published in the Dutch Government Gazette. The Advisory Group consists of expert representatives from the Healthcare Inspectorate, the Food Safety Authority, the Medicines Evaluation Board and the Central Committee on Research Involving Human Subjects. Its task is to issue advice on the legislation to be applied to individual products / product groups / substances belonging to a group of so-called borderline products. The reason that this Advisory Group was created is that the regulations in the field of market authorization and research with such products are complex and that it is not always clear which law is applicable. In such case it is also not clear which enforcement authority is competent to act in case of violations of the law. This is deemed undesirable by both the marketplace and the government authorities and the Advisory Group aims to change this.
Last week, the creation of the new Advisory Group on the Status of Borderline Products was published in the Dutch Government Gazette. The Advisory Group consists of expert representatives from the Healthcare Inspectorate, the Food Safety Authority, the Medicines Evaluation Board and the Central Committee on Research Involving Human Subjects. Its task is to issue advice on the legislation to be applied to individual products / product groups / substances belonging to a group of so-called borderline products. The reason that this Advisory Group was created is that the regulations in the field of market authorization and research with such products are complex and that it is not always clear which law is applicable. In such case it is also not clear which enforcement authority is competent to act in case of violations of the law. This is deemed undesirable by both the marketplace and the government authorities and the Advisory Group aims to change this.
Demarcation issues not new
Demarcation issues at the interface of the laws applicable to food products and medicinal products are not new. Already in a 2008 Letter to Parliament, the Dutch Health Minister reported that there was insufficient clarity about the demarcation between medicinal products and herbal remedies. The Minister reported the issue that a herbal remedy could also be a medicinal product within the meaning of the Dutch Act of Medicinal Products (in which the Medicinal Products Directive 2001/83 has been implemented). This is a consequence of the fact that the Act does not make a distinction between the origin of any active substance, which can be of human, animal, vegetable or chemical origin. When a herbal preparation qualifies as a medicinal product by function or by presentation, the Act on Medicinal Products is equally applicable.
Measures announced in the Letter of Parliament
At the time, the Health Minister did not consider it necessary to adjust the regulations to prevent demarcation issues between food products and medicinal products. He considered the criteria of “medicinal product by presentation” and “medicinal product by function” to be sufficiently clear in the first place. Secondly, he expected a beneficial effect from the list of permitted health claims that still had to be published back in 2008. He anticipated that products bearing such claims would not qualify as a medicinal product by presentation. He did, however, consider it desirable to improve cooperation between the Heath Care Inspectorate and the Food Safety Authority. This was implemented by adjusting the Decree on Supervision of Public Health, as a result of which the Food Safety Authority gained authority to enforce violations of the Act on Medicinal Products. Furthermore, it was stipulated that the structured consultation between the Heath Care Inspectorate, the Food Safety Authority and the Medicines Evaluation Board should be intensified, particularly regarding the discussion of the status of borderline products. One can say that this consultation in fact operated as an Advisory Group avant la lettre. Finally, it was determined that the already existing cooperation agreement between the Heath Care Inspectorate and Food Safety Authority had to be updated.
Demarcation issues more topical than ever
We now know that the clarification brought by the list of authorized health claims published in 2012 should not be overestimated. Nowadays many functional foods and nutraceuticals are marketed that claim medical properties. It quite often happens that it is not clear what is the applicable legislation to these products, because the boundary between health claims, disease risk reduction claims and medical claims is not immediately clear in all cases. Even when no specific claim is used, it can be debatable whether a product is a medicinal product by function or any other health product. It also happens that more or less the same products are marketed simultaneously as a medicinal product and as a foodstuff. Consider, for example, products containing glucosamine or St. John’s Wort. This means that food products containing the same active substance as medicinal products have not passed the prior testing for quality, safety and efficacy, which can be confusing for the consumer.
Consequences of product qualification
There are many examples of food products that were considered medicinal products by presentation. Consider, for example, the melatonin products which, after a remarkable turn in the Health Care Inspectorate policy, initially not but later on were considered to be medicinal products. More recently, a medical claim that Milk Thistle could prevent liver fattening was considered misleading. Furthermore, it was determined that a dietary supplement that would promote the natural immune system, would have a beneficial effect on heart and blood vessels and would help to treat fatigue qualified as a medicinal product. When such disputes are dealt with before the Civil Court, quite often fines are imposed for violations of the Act on Medicinal Products, prohibiting, among other things, the marketing and advertising of medicinal product without a marketing authorization. When these disputes are submitted to the Advertising Code Committee, usually a recommendation is made to no longer use such misleading information based on these regulations and the Code on Public Advertising of Medicinal Products. In order to prevent such enforcement activities, manufacturers of health products have every interest in knowing in advance how their product qualifies. They then know which regime applies to their product and can take this into account in their communication (advertising campaigns).
Working method Advisory Group
How does the Advisory Group work and which aspects are covered by its advice? Upon request of one of the competent authorities, the Advisory Group advises on the applicable legislation to individual products, product groups or substances. This advice gives a motivated indication of the law that applies to the opinion of the Advisory Group at the time of assessment and given the available information. “Available information” refers not only to factual information but also to applicable case law, both in the Netherlands and abroad. The advice will be presented to the Healthcare Inspectorate, the Food Safety Authority, the Medicines Evaluation Board and the Central Committee on Research Involving Human Subjects, who will subsequently respond to this within four weeks if so desired. If the responses give cause to do so, the Advisory Group reconsiders the advice and if necessary adjusts it. The advice is then recorded in its database, which is managed by its secretariat. There is also a possibility that the Advisory Council will not reach agreement on the applicable legislation. In such a case no advice is issued; this is also recorded in the database. The Advisory Group can also change its advice in the event of changes in the legal framework. The competent enforcement authorities must report those change to the Advisory Group, which registers an amendment to a previous advice in its database, if and when applicable. For clarity, the advice assesses neither the efficacy, safety or efficacy of medical products nor the content of any research protocols.
Added value Advisory Group
Can it be expected that the Advisory Group will make a substantial contribution to demarcation issues? In itself, the intensified co-operation between all the authorities involved, at market entry rather than upon enforcement during marketing, is to be welcomed. For example, in practice, food business operators also benefit from the working agreements between the Council on Advertising Health Products and the Food Safety Authority, on the basis of which health products bearing a “stamp” of said Council are in principle not exposed to enforcement actions by the Food Safety Authority. However, it appears from the currently published agreement between the parties involved in the Advice Group that only the enforcement authorities and not the individual manufacturers can request advice from the Advisory Group. This appears to be a missed opportunity, as is the fact that the efficacy of medicinal products remains explicitly outside the Advisory Council’s assessment. To qualify the essential character of a health product, efficacy is one of the essential factors. Furthermore, it is noteworthy that the Advisory Group does not seem to address the issue of medical devices, while this qualification is closely linked to that of medicinal or food products. For example, cranberry pills against bladder infection qualify as medical devices and osmotic laxatives can be either medicinal products or medical devices.
Advice on market access for health products
For the time being, manufacturers of health products will continue to rely on private advice from qualified advisors on market access for health products. Nevertheless, the body of opinions to be produced by the Advice Group can be a valuable source of information, provided that its database will be publicly searchable. The rules of procedure of the Advice Group, which are yet to be made available, will have to provide clarity in this respect.
The new Organic Regulation: from farm to fork approach reinforced
Posted: May 9, 2018 | Author: Jasmin Buijs | Filed under: Authors, Information, organic | Comments Off on The new Organic Regulation: from farm to fork approach reinforced The organic sector has developed from a niche market to one of the most dynamic sectors of EU agriculture. To recall some numbers provided by the European Commission, the amount of land used for organic farming grows at around 400,000 hectares a year. The organic market in the EU is worth around €27 billion, some 125% more than ten years ago. The EU encourages more farmers into the organic sector and aims to increase consumers’ trust in certified organic products to further boost those numbers. For some background on the regulatory framework, we refer to our earlier blogpost on the Organics Regulation. As already announced in that post, the current Council Regulation (EC) No 834/2007 needs an update as it is based on practices of over 20 years ago. The first proposal for a new EU Regulation on organic production dates back to 2014. Last month, the European Parliament Committee on Agriculture and Rural Development gave green light to the new Organic Regulation. This new regulation aims to guarantee organic production throughout the supply chain by phasing out the many exemptions that are allowed under the current Regulation, such as the use of non-organic seeds as further covered below. In other words, the new regulation shifts from an à la carte system of exceptions to a set menu of harmonized rules. This contribution sets out the most important changes by answering relevant questions in the light of the new Organic Regulation.
The organic sector has developed from a niche market to one of the most dynamic sectors of EU agriculture. To recall some numbers provided by the European Commission, the amount of land used for organic farming grows at around 400,000 hectares a year. The organic market in the EU is worth around €27 billion, some 125% more than ten years ago. The EU encourages more farmers into the organic sector and aims to increase consumers’ trust in certified organic products to further boost those numbers. For some background on the regulatory framework, we refer to our earlier blogpost on the Organics Regulation. As already announced in that post, the current Council Regulation (EC) No 834/2007 needs an update as it is based on practices of over 20 years ago. The first proposal for a new EU Regulation on organic production dates back to 2014. Last month, the European Parliament Committee on Agriculture and Rural Development gave green light to the new Organic Regulation. This new regulation aims to guarantee organic production throughout the supply chain by phasing out the many exemptions that are allowed under the current Regulation, such as the use of non-organic seeds as further covered below. In other words, the new regulation shifts from an à la carte system of exceptions to a set menu of harmonized rules. This contribution sets out the most important changes by answering relevant questions in the light of the new Organic Regulation.
Which products are covered under the new Organic Regulation?
Similar to the current Organic Regulation, the new Organic Regulation applies to live and unprocessed agricultural products, including seeds and other plant reproductive material and processed agricultural products used as food and feed. Processed products can be labelled as organic only if at least 95% by weight of their ingredients of agricultural origin are organic. Unlike the current Organic Regulation, the new Organic Regulation also covers certain other products closely linked to agriculture. Those products are listed in Annex I and include, among others, salt, essential oils, cork, cotton, and wool. Other products may be added in future.
To what extent can non-organic seeds still be used?
Derogations that allow non-organic seeds to be used in organic production will expire in 2035. A couple of measures will be taken to increase the organic seed supply and to help it meet high demands before that time. First of all, Member States shall establish a database of organic plant reproductive material as well as national systems that connect organic farmers with suppliers of organic reproductive material. Secondly, the use non-organic seeds may temporarily remain allowed if the collected data demonstrates insufficient quality and quantity of the organic reproductive material. Also, to meet the demands of organic seeds, organic heterogeneous material is explicitly allowed for in Article 13 of the new Organic Regulation and production criteria for organic varieties are adapted taking into account the specific needs and constraints of organic production. The derogations may be phased out earlier than 2035 or extended based on a report due in 2025, which will examine the situation of plant reproductive material on the market.
Are mixed farms still allowed under the new Organic Regulation?
While the initial proposal of the Commission proposed to ban the production of organic products and conventional products at the same farm, mixed farms continue to exist under the new Organic Regulation. Mixed farms are allowed provided that the two production activities are effectively separated into clearly distinct production units. This means, among others, that inputs for production as well as the final products must be separated to avoid contamination and potential fraud. Also, the two production activities should involve different livestock species and plant varieties.
What measures must be taken to avoid contamination from non-authorized substances?
EU thresholds for conventional products automatically apply to organic ones too. Stricter thresholds for non-authorized substances in organic products are not introduced. This would include high costs, especially for small farmers, to control for example contamination from neighbouring conventional farming. Instead, food business operators are obliged to take precautionary measures to avoid contamination. The responsibility and accountability of organic producers will thus be emphasized. Final products are not allowed to bear the organic label when the contamination was deliberate or caused by irresponsible food business operators that failed to take precautionary measures. Meanwhile, Member States remain at liberty to set specified thresholds for non-authorized substances in organic products, provided that these national rules will not affect the trade of organic products that are legally placed on the market in other Member States. Based on a report due by the end of 2024, anti-contamination rules and national thresholds may be further harmonized in future.
How does the new Organic Regulation ensure the high quality of organic products?
Rather than moving all the control provisions into the regulation on official controls for food and feed, as initially proposed by the Commission, specific rules will apply to the control of organic farming. Organic production refers to the use of production methods that contribute to the protection of the environment, animal welfare, and rural development. Risk-based checks will therefore not be limited to final products but take place along the supply chain to guarantee that organic products are truly organic. Physical on-site checks will take place at least annually; the on-site check may be reduced to once every two years if the food business operator has been fully compliant for three years. In the Netherlands, those inspections are carried out by SKAL as the the designated Control Authority responsible for the inspection and certification of organic companies.
What does the new Organic Regulation mean for products imported into the EU?
Under the current Organic Regulation, organic products produced in third countries are allowed on the EU market when the organic standards of the exporting country are similar to EU rules. This means that organic products are in fact regulated by over 60 different standards. Under the new Organic Regulation, all imported products will have to comply with EU standards. Taking into account the date of application of the new Organic Regulation as well as the transitional period granted for imported products, this rule will only apply as from 2026. Moreover, certain exceptions are introduced to avoid disruptions of supply on the EU market. First of all, the Commission is empowered to grant specific authorization for the use of products and substances in organic production in third countries with specific climatic and local conditions, which therefore cannot comply with the new requirements. This exception also applies to organic production in the EU’s outermost regions, such as the Nordics. Secondly, equivalent production methods in third countries could be recognised under trade agreements.
Conclusion
The new Organic Regulation promises to further boost EU organic production. Measures to increase the supply of organic seeds and animals, allowing mixed farming, and no harmonized thresholds for non-authorized substances aim to attract more farmers into organic production. Meanwhile, measures are taken to increase consumers’ trust in organic products, such as through risk-based checks along the supply chain and the switch from the principle of equivalence to an EU single set of rules for imported products. We expect this to be a major improvement for the algae sector, that suffers unfair competition from Asian countries, where organic standards are not necessarily the same as in the EU.
It takes some more years to see to what extent the Regulation will live up to its promises. While the Regulation itself only becomes applicable from 2021, many rules are subject to further implementation depending on the development of the sector. This illustrates the dynamic character of the organic sector, which creates many opportunities for food business operators active in this field.
Moving in high spirits towards crystal clear alcoholic beverage information
Posted: April 7, 2018 | Author: Jasmin Buijs | Filed under: Authors, Food, Information | Comments Off on Moving in high spirits towards crystal clear alcoholic beverage information Last month, the European alcoholic beverages sectors handed over to the European Commission a self-regulatory proposal on the provision of nutrition information and ingredients listing. Article 16(4) of the Food Information for Consumers (FIC) Regulation exempts alcoholic beverages containing more than 1.2% by volume of alcohol from the mandatory list of information and nutrition declaration. Nevertheless, it also attributes the Commission with the task to investigate whether and to what extent alcoholic beverages should nevertheless be covered. Last year, the Commission established in its report, amongst others, that the provision of nutrition information and ingredients listing could help consumers to make informed choices about what and how much to drink. The alcoholic beverages sectors have been asked to present a self-regulatory proposal to respond to consumers’ expectations as they have done last month.
Last month, the European alcoholic beverages sectors handed over to the European Commission a self-regulatory proposal on the provision of nutrition information and ingredients listing. Article 16(4) of the Food Information for Consumers (FIC) Regulation exempts alcoholic beverages containing more than 1.2% by volume of alcohol from the mandatory list of information and nutrition declaration. Nevertheless, it also attributes the Commission with the task to investigate whether and to what extent alcoholic beverages should nevertheless be covered. Last year, the Commission established in its report, amongst others, that the provision of nutrition information and ingredients listing could help consumers to make informed choices about what and how much to drink. The alcoholic beverages sectors have been asked to present a self-regulatory proposal to respond to consumers’ expectations as they have done last month.
In their proposal, the alcoholic beverages sectors elaborate on the details on the communication of the nutrition information, the list of ingredients, and the means of delivering this information to consumers. The sectors aim to provide consumers with meaningful information while preventing to create new burdens for SMEs. In this blog we give a short overview of the proposal, which should be read together with the sector-specific annexes for beer, cider, spirits and wine that allow for sector-specific implementation of the proposal.
According to the FIC Regulation, nutrition information must be given per value of 100 ml of the product and may additionally be provided per portion size. Nutrition information involves the energy value and the amounts of fat, saturates, carbohydrate, sugars, protein and salt, but may also be limited to the energy value only. The spirit sector emphasizes in its annex that it is most meaningful for consumers to provide the information in portion size and adds that the energy value per 100 ml might be misleading, as spirits are never served in this quantity. To provide accurate nutrition information, food business operators may carry out analysis on their products or they can use generally established and accepted data. The alcohol beverages sectors stress the importance of the latter option due to the changing nature of certain alcoholic beverages as they age (wine) and depending on the harvest (e.g. cider). Moreover, said data will also reduce the burden for analysis by SMEs. The wine and spirits sector have already provided such a toolkit in their annexes to the proposal, using average values of typical and characteristic wines. For instance, 100 ml of brut, sparkling wine provides 70 kcal.
As for the list of ingredients, Article 20 of the FIC Regulation excludes food additives or food enzymes used as processing aids from this list. The wine sector specifies that this means that only additives permitted for wine-making not considered processing aids during the wine-making process shall be included in the list of ingredients. Moreover, the sector notes that natural substances used to adjust the grape composition (acidity and natural sugar content) do not have to be listed; those substances only aim to restore the basic balance and composition where harvest conditions are not optimal. The wine annex is already rather concrete in the sense that it shows in its appendix I a list of oenological compounds that, when used, will have to be included in the list of ingredients.
 Lastly, the proposal embraces new information and communication technologies to inform the consumer on the nutrition declaration and list of ingredients. This is in line with recital 51 of the FIC Regulation, according to which food information rules should be able to adapt to, amongst others, a rapidly changing technological environment. Next to traditional on label information, off-label information that can be accessed via a web-link, QR code, bar code or other direct means of using smart technologies are suggested in the proposal. The wine sector refers in its annex for example to the website of ‘Wine in Moderation’ as a tool to comply with labeling requirements by off-label means. The alcoholic beverages sectors propose to leave it up to the food business operators how to display the information.
Lastly, the proposal embraces new information and communication technologies to inform the consumer on the nutrition declaration and list of ingredients. This is in line with recital 51 of the FIC Regulation, according to which food information rules should be able to adapt to, amongst others, a rapidly changing technological environment. Next to traditional on label information, off-label information that can be accessed via a web-link, QR code, bar code or other direct means of using smart technologies are suggested in the proposal. The wine sector refers in its annex for example to the website of ‘Wine in Moderation’ as a tool to comply with labeling requirements by off-label means. The alcoholic beverages sectors propose to leave it up to the food business operators how to display the information.
The alcoholic beverages sectors commit themselves to report on implementation in March 2021. The proposal will now be assessed by the Commission. Green light will allow for high spirits to make information on alcoholic beverages crystal clear. However, all four sectors (beer, cider, spirits and wine) must be working hard on more concrete implementation guidelines that can be used by their member companies, who have to do the actual job. For the time being, the wine and spirits sector so far have elaborated the most concrete proposals. We will continue to monitor and report on further developments in this field.
Natural foods – what’s in a name?
Posted: March 20, 2018 | Author: Karin Verzijden | Filed under: Authors, Food, Nutrition claims | Comments Off on Natural foods – what’s in a name? In their search for healthy foods, consumers nowadays often favour natural food products. But what is “natural” after all? Various interpretations could be given to this term. Amongst those figure unprocessed foods, foods without any chemical ingredients and organic foods. In reality, there is no harmonized concept of “natural foods” under EU food law. Food business operators should therefore mainly rely on the general principles of food information. In addition, they can use the nutrition claim “natural(ly)”, if and when applicable. Finally, they can turn to some specific pieces of EU legislation implementing the concept of “natural” for specific product an to a new ISO standard. This post aims to investigate what are the options for using the term “natural” based on the EU framework.
In their search for healthy foods, consumers nowadays often favour natural food products. But what is “natural” after all? Various interpretations could be given to this term. Amongst those figure unprocessed foods, foods without any chemical ingredients and organic foods. In reality, there is no harmonized concept of “natural foods” under EU food law. Food business operators should therefore mainly rely on the general principles of food information. In addition, they can use the nutrition claim “natural(ly)”, if and when applicable. Finally, they can turn to some specific pieces of EU legislation implementing the concept of “natural” for specific product an to a new ISO standard. This post aims to investigate what are the options for using the term “natural” based on the EU framework.
The most important principle under EU food law is that food information should not be misleading, in particular as to its characteristics, its nature and its composition. As such, it is prohibited to suggest that a food has particular characteristics, whereas in fact all similar foods possess such characteristics. For instance, certain additives, such as sweeteners or acids, may have a natural source, but the EU Additives Regulation does not make a distinction between “natural” or “artificial” (chemical) additives. Therefore, stating that a food product only contains natural preservatives could be misleading. The industry seems to have found its way around this issue. Instead of declaring an additive using the applicable E-number (for instance E 250 for sodium nitrate), the description of a natural compound having a similar technical function is used (for instance chard, having a similar preservative effect to sodium nitrate).
Where a food product naturally meets the conditions laid down in in the EU Claims Regulation for the use of a nutrition claim, the term “natural(ly) may be used as a prefix in the claim. For instance, if at least 20 % of the energy of the food product is naturally provided by protein, the claim “naturally high in protein” could be made regarding such product. Likewise, if a food product naturally contains at least 6 g fibre per 100 g product, the claim “naturally high in fibre” could be made. These claims should all relate to end products presented to consumers. As a B2B supplier of ingredients, you should therefore make the reservation to your clients that the admissibility of these claims depends on the mix of ingredients present in the end product.
The EU Flavouring Regulation constitutes a good example of targeted legislation with specific provisions on the use of “natural”. For instance, if the term “natural” is used to describe a flavour, the flavouring components should be entirely of a natural origin. Nevertheless, the term “natural flavouring” can be used in a situation where a clear relationship between different source materials in the flavouring component and the overall flavour-profile is lacking. For instance, an umami flavouring preparation consisting of certain vitamins and minerals in combination with certain fermentation media can still be labelled as “natural flavouring”.
The Dutch Advertising Code Committee (DACC) demonstrated in one of its decisions last year that the rules on the use of the term ‘‘natural’’ under the EU Flavouring Regulation can also be applied by analogy. The claim in the case in question related to BenBits chewing gum advertised as being “100 % natural”. A competitor denied that all ingredients used in this chewing gum were of a natural character. In deciding the dispute, the DACC took the expectation of the average, reasonably well-informed consumer as a starting point. Such consumer would expect the term “natural” to cover both the ingredients and the preparation processes used. BenBits could not demonstrate that the xylitol used in its product had been manufactured in accordance with one of the traditional preparation processes referred to in the EU Aroma Regulation, such as fermentation. The use of the claim “100 % natural chewing gum” was therefore considered misleading.
Recently, a new ISO standard was made available, containing technical criteria for food ingredients to be considered as natural. This standard is intended for use in B2B communications and conformity thereto helps to assure a level playing field and fair practices. According to this standard, food ingredients shall be considered as natural when certain criteria regarding both their source materials and their ways of processing are met. When a food product consists of various ingredients, for each ingredient these cumulative criteria should be met. An informative decision three in this standard helps its user decide whether or not a food ingredient is to be considered natural.
At Member State level, such as in the UK and in France, national guidance has been formulated for the use of the term “natural”. For instance, the UK Food Standard Agency issued guidelines for the use of this term and in France the Directorate General on Competition developed similar guidelines specifically targeting dairy products. These Guidelines, as well as relevant EU and national case law will be discussed during the Vitafoods session planned on 16 May from 11.20 – 12.00 AM. Hope to see you there!
Nutrition & cognition – how to communicate on innovative food products in this particular field?
Posted: December 13, 2017 | Author: Karin Verzijden | Filed under: Authors, Food, Food Supplements, Health claims, Information | Comments Off on Nutrition & cognition – how to communicate on innovative food products in this particular field? Consumers across all demographics are increasingly concerned about cognitive health and performance. For that reason, Food Matters Live, held in London from 21 – 23 November last, dedicated one of its Seminars to the exploration of new R&D advancing the understanding of nutrition and cognitive health and performance. I was asked to give a presentation on meeting standards for cognitive claims, of which you will find a summary below. You will note that in addition to the system of currently authorised claims, I will explore its flexibility, as well as the options outside its scope.
Consumers across all demographics are increasingly concerned about cognitive health and performance. For that reason, Food Matters Live, held in London from 21 – 23 November last, dedicated one of its Seminars to the exploration of new R&D advancing the understanding of nutrition and cognitive health and performance. I was asked to give a presentation on meeting standards for cognitive claims, of which you will find a summary below. You will note that in addition to the system of currently authorised claims, I will explore its flexibility, as well as the options outside its scope.
FIC Regulation
The general framework for health claims is contained both in the FIC Regulation and in the Claims Regulation. The FIC Regulation embodies the principle of fair information practises. According to this principle food information should not be misleading as to the characteristics of the food, for example by attributing it effects it does not possess. Furthermore, the FIC Regulation prohibits any medical claims to be made in connection with food products. A medical claim is to be understood as any claim targeting the prevention or treatment of a particular disease. It is for instance not permitted to state that a food supplement alleviates the symptoms of rheumatoid arthritis.
Claims Regulation
The Claims Regulation lays down the very concept of a health claim, being a voluntary message in any form that states or suggests that a food has particular characteristics. Basically, a health claims conveys the message “What does the product do?” Health claims can only be made with regard to a particular nutrient that has been shown to have a beneficial nutritional of physiological effect. Such nutrient should be present in the end product in a form that is bio available and to such extent that it produces the claimed effect. The scope of the Claims Regulation includes all commercial communications regarding food products to be delivered to the final consumer. Based on the Innova / Vital decision of the ECJ, it was clarified that such final consumer can also be a health care professional.
Legal framework for cognitive claims
Currently, authorised claims for cognition can be linked to iodine, iron and zinc. For all of these compounds, the claim “contributes to the normal cognitive function” can be made. In addition, for iodine the claim “contributes to the normal functioning of the nervous system” is available and for iron a claim specifically targeting children can be made. The conditions of use for these claims are calculated on the reference intake (“RI”) applicable to each mineral. As such, a distinction is being made between solids (15 % RI) and fluids (7.5% RI). For instance, in order to allow a cognition claim linked to iron, the end product should at least contain 2.1 mg iron / 100 g or 1.05 mg / 100 ml. Any claim should refer to a food product ready for consumption, prepared in accordance with the manufacturer’s instructions.
Flexibility in wording
In practise, I see that many food business operators try to reword the authorised claims, as they are not considered to be a major add-on. In the Netherlands, this practise has been facilitated by the Council for the Public Advertising of Health Products (Keuringsraad KOAG-KAG), who has published a list of alternative, authorized claims. For instance, for a claim on zinc, the Dutch translation of the wording “contributes to a regular problem-solving ability” is permitted as an alternative. Regarding a claim on iodine, you could think of “plays an important role in mental activity.” Furthermore, it is also permitted to state that a food product containing the required minimum of iron “contributes to regular intelligence”. Now we are talking!
Examples found in practise
An internet search for products targeting cognition revealed that many of them are not linked to the EU authorized claims at all. For instance, the company Flora Health is marketing the food supplement ginkgo biloba, claiming that it “helps to enhance cognitive function and memory in an aging population.” Also, the food supplement Mind Focus containing various vitamins, minerals and green tea extract from the company Bio Fusion was found, which allegedly “improves mind focus and concentration instantly”. Furthermore, the green oat product Neuravena was found, regarding which five clinical studies confirm it benefits to cognitive function. How can this be explained? The first two examples contain claims that in the EU would no doubt qualify as medical claims and as such are prohibited.
Non-EU products and options outside cognitive claims framework
Products that do not comply with EU standards may originate from other countries or territories that are subject to a different regulatory regime than applicable in the EU. Without endorsing any claims made, it was found that the gingko and Mind Focus products originated from Canada and the US respectively. The same is not true for the product Neuravena, as its manufacturer Frutarom claims to be a “global manufacturer of health ingredients backed by the science, and supported with documentation and the regulatory compliance our customers demand.” The difference here is that Neuravena is not advertised on a commercial setting targeting end consumers, but in a scientific portal. The website even contains a disclaimer to that extent. In a non-commercial, purely scientific environment, the Claims Regulation is not applicable. This allows FBO’s, provided that the same website does not contain a click through ordering portal, to communicate on their R&D and cognition even outside the authorised EU framework.
Conclusion
The EU authorised claims for cognition are limited in number and scope. Several EU Member States offer considerable flexibility in wording, which makes the use of claims much more appealing. Furthermore, in a science-based context, the Claims Regulation is not applicable, which allows you plenty of opportunity to communicate your latest R&D on nutrition & cognition, provided that the message is strictly scientific and not commercial.
The EU Tofutown case – don’t mess around with dairy product names
Posted: July 31, 2017 | Author: Karin Verzijden | Filed under: Authors, Enforcement, Food, Information | Comments Off on The EU Tofutown case – don’t mess around with dairy product names The European Court of Justice (ECJ) recently took its decision in the TofuTown case, providing clarity on the interpretation of the Agricultural Product Standards Regulation (APS Regulation). In short, this case dealt with the question: “Is it possible to use regulated product names for new product types?” The answer to this question is a clear “no”. This came as quite a shock to the emerging vegetarian and vegan market. Many companies marketing alternative protein products use traditional product names for their dairy replacements. They may have to change these names, as they are no longer in line with the view of the highest EU Court.
The European Court of Justice (ECJ) recently took its decision in the TofuTown case, providing clarity on the interpretation of the Agricultural Product Standards Regulation (APS Regulation). In short, this case dealt with the question: “Is it possible to use regulated product names for new product types?” The answer to this question is a clear “no”. This came as quite a shock to the emerging vegetarian and vegan market. Many companies marketing alternative protein products use traditional product names for their dairy replacements. They may have to change these names, as they are no longer in line with the view of the highest EU Court.
Initiator: Verband Sozialer Wettbewerb
The TofuTown case was initiated by the German unfair competition association Verband Sozialer Wettbewerb (“VSW”). This activist organisation was also at the origin of the Innova Vital case, clarifying the scope of the EU Claims Regulation. Based on this decision, we now know that the Claims Regulations not only targets commercial communications addressing final consumers, but under certain circumstances, it also addresses communications made to health care professionals. VSW initiated the present case against the German company TofuTown, as it claimed the promotion of strictly plant-based products under denominations such as “Soyatoo Tofubutter”, “Pflanzenkäse” and “Veggiecheese” infringed the EU competition rules.
Main rule of Agricultural Product Standards Regulation
The legal framework of this dispute is constituted by the APS Regulation, containing detailed agricultural products definitions in various sectors, such as cereals, olive oil, wine, milk and milk products. The main rule following from the APS Regulation is that the names of regulated products may not be used for other products. TofuTown had argued, in essence, that this principle is outdated. In the past, such rule may have been justified to protect consumers against manufacturers distributing cheap products as dairy products, thereby gaining a competitive advantage. Nowadays, consumer awareness has changed considerably and many consumers want to be informed about alternatives to dairy products. Using regulated dairy names for plant-based alternatives is not meant to mislead consumers. Quite to the contrary, such use enables to inform the consumer on plant-based origin of their products as opposed to the animal derived dairy products they intend to replace. The ECJ did not agree with this argument. It is nevertheless worthwhile to investigate if any other argument could escape the strict product name regime embodied in the APS Regulation.
Room for manoeuvre in APS framework?
On the face of it, there is. The Commission is empowered to adopt delegated acts concerning exemptions to the strict product definitions laid down in the APS Regulation, provided these shall be strictly limited to demonstrated needs resulting from evolving consumer demand, technical progress or the need for product innovation (see article 78.3 APS Regulation). Tofutown’s plea for informing consumers about plant-based alternatives to dairy products seems to fit in seamlessly in this derogation. However, for the time being, there is no such delegated act, so that it is worth while exploring if there any further exceptions to the strict product names principle embodied in the APS Regulation.
Composite products
In fact, two of these exceptions are being mentioned in the TofuTown case. In the first place, designations for milk products may also be used in association with words designating composite products. The condition precedent is that no part takes the place of any milk constituent. Furthermore, milk should also be the essential part of such composite product, either in terms of quantity or for the characterisation (see Annex VII belonging to the APS Regulation, in particular Part III, nr. 3 thereof). An examples of such composite product names is chocolate milk.
Traditional usage
Another exception to the system of strict product names relates in the first place to products the exact nature of which is clear from traditional usage. In the second place it covers designations that are clearly used to describe a characteristic quality of the product. The Commission has drawn up a limitative list of such product names that can be found here. Examples of such product names are the following:
- UK: horseradisch cream, cream crackers and coconut milk
- France: almond milk (lait d’amande) and oat cream (crême d’avoine)
- Spain: almond milk (leche de almendras)
- Netherlands: butter beans (boter bonen) and cacao butter (cacao boter)
This list has a highly cultural character and does not allow for translations of the product names mentioned therein into other Member States languages. As such, it would be prohibited to market almond milk in or any other EU country than France and Spain. The same goes for butter beans outside the Netherlands.
Practical consequences
It could be argued that names like “Tofubutter” and “Veggiecheese” are clearly used to describe a characteristic quality of the product. However, as long as they are not mentioned on the Commission exemption list, these cannot be legally used for marketing plant-based alternatives to dairy products. More in general, the practical consequences of the TofuTown decision are expected to bring about serious restrictions for marketing plant-based products. Is this to be considered a setback for the consumer or to the contrary, will this prevent any consumers being misled? In fact, this is besides the point, as based on this decision, the enforcement authorities, like the Dutch Food Safety Authority (“NVWA”) in the Netherlands, will be authorised to prohibit further use of strictly regulated product names for their vegetarian peers. It will be interesting to see though if any enforcement actions will take place based on the mere product name. And also, will this be in the interest of the educated consumers of today? That’s certainly not beside the point.
