Posted: February 5, 2025 | Author: Karin Verzijden | Filed under: Advertising, alternative protein, Authors, cultivated meat, Food | Tags: Advertizing |
 The Dutch Dairy Organisation (NZO) launched a campaign starring young farmers. This initiative took shape through a website and a TV commercial. It was however not entirely clear if the primary purpose of this campaign was to boost the image of young farmers or to boost dairy sales. In a decision provided at the end of last year, the Appeal Board of the Dutch Advertising Code Committee (ACC) explains why transparency around sponsorship of a nonprofit campaign is necessary. It also clarifies when a party is considered a co-advertiser.
The Dutch Dairy Organisation (NZO) launched a campaign starring young farmers. This initiative took shape through a website and a TV commercial. It was however not entirely clear if the primary purpose of this campaign was to boost the image of young farmers or to boost dairy sales. In a decision provided at the end of last year, the Appeal Board of the Dutch Advertising Code Committee (ACC) explains why transparency around sponsorship of a nonprofit campaign is necessary. It also clarifies when a party is considered a co-advertiser.
Website
Under the heading “farmers love cows”, the website states, among other things, “As young farmers of the Netherlands, we would like to tell people that we take good care of our animals. You can see that in these commercials.” The TV commercial is then shown, followed by the text: “What dairy cows want”. What follows is an enumeration of cows’ wishes with pictures: “being able to walk around freely”, “always having enough water and feed”, “milked with care”, “always having fresh air” and “chilling out”. Following this, under the heading “what our farmers do” are short stories from three farmers. At the bottom of the page, it is stated “This campaign was created with the support of the NZO”. The NZO logo is provided next to this announcement.
TV commercial
The TV commercial shows images of young farmers, working on the farm, and of dairy cows, both in the barn and in the meadow. The voice-over in the TV-commercial says: “We farmers take good care of our dairy cows. They can walk around if they want to. Brush themselves if they want to. Chill out if they want to. And eat and drink when they want. They are milked with care. And there is always fresh air. See, that makes them happy. And if the cow is happy, so am I. Farmers love cows. This is a message from the Dutch Young Farmers Association.” At the end of the television commercial, both the web address of the campaign and the logo of the Young Farmers Association is mentioned.
Message on behalf of whom?
Both the website and the TV commercial are displayed by the Dutch Young Farmers Association. This is an organisation that represents the interests of agricultural young people in The Hague and Brussels, with the aim of helping them with personal and business development. However, in the background, the NZO is also involved. The website states this in so many words. In the TV commercial, this appears indirectly, namely by reference to the website of the campaign. In first instance, the ACC did not consider NZO as a co-advertiser. Wrongly, according to the complainant, because NZO was in fact instrumental in the creation of this campaign.
Image campaign for young farmers or statement on behalf of NZO?
Advertising is a broad concept, covering not only the recommendation of products and services, but also of ideas. It makes a difference to consumers’ interpretation whether these ideas are proclaimed by an idealistic or a commercial organisation. Is this an idealistic campaign to boost the image of young farmers or a commercial expression by an organisation that aims to promote dairy production and marketing on behalf of its members? If there is no clarity on the involvement of NZO, this could be interpreted as a lack of essential information. This could potentially make this campaign misleading.
Misleading campaign?
To start with the latter, the Appeal Board distinguishes between the website and the TV commercial in its assessment of whether the campaign would be misleading. Indeed, the Appeal Board rules that consumers should be informed about the commercial aspect of the message. This should prevent consumers from putting the TV commercial in the wrong context, so that they will see it exclusively as an image campaign for young farmers. If the consumer does not recognise the underlying commercial interest of NZO, what is left is a romanticised image of how young farmers treat and love cows. And it is not unlikely this will boost sales of dairy products. The Appeal Board considers it plausible that the lack of information about the fact that the campaign is (partly) a commercial expression in the interest of the dairy sector may lead the average consumer to buy dairy products, which they would not have done otherwise. Information about this interest is therefore essential information for the average consumer.
Lack of “essential information”
Because the TV commercial omits information about the involvement of NZO or other information about the underlying commercial interest, there is a lack of essential information as referred to in article 8.3 of the Dutch Advertising Code (NRC). Like the ACC, the Appeal Board rules that the campaign is unfair for that reason within the meaning of Article 7 NRC. This finding is without prejudice to the right to freedom of expression by the Young Farmers Association and NZO. They are allowed to propagate a viewpoint regarding young farmers in the television commercial but must also comply with the requirement that no unfair advertising is made. Freedom of expression as guaranteed in Article 10(2) of the European Convention on Human Rights does not imply the freedom to engage in unfair advertising.
The NRC’s provisions on unfair advertising are a direct implementation of the corresponding provisions of Dutch statutory laws on this point. It thus concerns an exception to freedom of expression provided by law. Moreover, the restriction in this case relates exclusively to the disclosure of the underlying commercial interest and not to the idea itself. NZO and the Young Farmers Association therefore fail on this point as far as the TV commercial is concerned. This does not apply to the website. The Appeal Board rules on this point that the announcement that the campaign was created “with the support of NZO” makes it sufficiently clear that the dairy industry as such is involved in and supports this campaign.
NZO co-advertiser
The NZO is closely involved in the TV commercial, as it (i) financed the production thereof, (ii) registered the domain name of the campaign website and (iii) took care of the procurement of airtime of the TV commercial. As mentioned above, promotion of dairy products should be seen as the primary objective of the campaign. With NZO on the one hand enabling the campaign and on the other hand promoting its own commercial interest, the Appeal Board sees sufficient reason to consider NZO co-responsible for the content of the TV commercial. NZO actively helped the Young Farmers Association to advertise the dairy sector. This constitutes the promotion of dairy products by or on behalf of an advertiser, whether or not with the help of third parties as referred to in article 1 NRC. This gives the NZO its own responsibility to ensure that the campaign complies with the NRC, which it failed to do. That unfair advertising is made through the TV commercial is therefore partly attributed to NZO.
What can we learn from this decision?
This decision shows that it is perfectly permissible to convey an idealistic message by or on behalf of a commercial organisation. But it must be transparent who is proclaiming this message. This lesson applies equally to conventional and alternative dairy and meat products. If manufacturers of alternative proteins conduct an idealistic campaign in which they emphasize that manufacturing their products does not involve animal suffering, it must be clear who is sending this message. These are just the rules of fair advertising, which benefit to all of us. Just like alternative protein options.
Picture sourced from the website https://boerenhoudenvankoeien.nl.
Posted: October 7, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Authors, clean meat, cultivated meat, Food, Information |
 On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
Legal names can be set, but the French Decree does not contain legal names
The ECJ starts the motivation of its decision with acknowledging that the FIC Regulation leaves room to adopt legal names at Member State level where such do not exist at EU level. Where legal names are set, these cannot be used for products not complying with the specifications of that name. As an example, the ECJ refers to the term ‘meat’, which is legally defined as ‘the edible parts of animals’. A food not containing such parts can therefore not use the name ‘meat’, even if it is accompanied by specifying terms such as ‘vegetarian’. The same applies to milk and certain milk products, for which the legal name is laid down in the COM Regulation. Indeed, as we know well from the Tofutown decision, names such as ‘plant-based milk’ are a no-go.
According to AG Capeta, the French Decree under attack established legal names. This was done on the one hand by establishing a list of meaty names of which the use is prohibited for the designation of their plant-based counterpart (such as steak), and on the other hand by authorizing the use of certain meaty names for foods containing vegetable proteins provided that they do not exceed a certain proportion (such as cordon bleu (maximum 3,5 % vegetable protein)).
The ECJ ruled however differently. In the first place, it recalls that the French authorities themselves rejected the hypothesis that Decree No 2022-947 lays down a legal name. Therefore, the learnings from the Tofutown decision cannot be applied to the case at hand. In the second place, it also states that legal names must, according to the definition thereof in the FIC Regulation, be defined in order to designate a foodstuff. The adoption of a legal name thus means associating a specific expression with a given food. This is done by setting certain conditions, especially with regard to the composition of the food. The French Decree contains a measure prohibiting the use of certain meaty names, which are not legally defined by the Decree, for plant-based foods. This is not the same.
Use of customary and descriptive names fully harmonized
Given that there are no legal names for plant-based foods at EU level and neither in France as far as is known to the ECJ based on the file of the case, plant-based foods must be indicated by their customary name or descriptive name. Where a customary name is the accepted name of the food that does not need further explanation, a descriptive name must explain the main characteristics of the food.
 Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
No distinction between domestic and imported products
Where the AG made in her opinion a distinction between rules covering domestic production and rules covering production abroad, this topic was no longer covered in the ECJ’s preliminary ruling. The question whether the French Decree could only apply to foods manufactured in its territory was initiated by the highest French administrative court (“Conseil d’Etat”), but became redundant since the ECJ came to the conclusion that national measures regulating or prohibiting the use of meaty names for plant-based products (other than by means of legal names) is not allowed in the first place.
We nevertheless conclude from the case that since the limitative legislation applying at a national level is not considered in conformity with Union legislation, this surely goes for national legislation applying similar restrictions on a Union level. This is justified by the fact that the ECJ recalls at the beginning of its decision the two paramount principles of Union legislation. In addition to consumer protection, this is also the smooth functioning of the internal market.
Outlook for the alternative protein sector a whole
Now the ECJ has given its interpretation of the FIC Regulation in response to the questions of the French referring court, it is now up to the latter to decide the dispute at national level in accordance with the ECJ’s preliminary ruling.
It should be noted that the ECJ’s ruling is similarly binding on other national courts or tribunals before which a similar issue is raised. This means that the prohibition to enact national measures regulating or prohibiting the use of meaty names in the absence of legal names in principle also applies to other meat analogues such as cultivated meat and those produced by precision fermentation. Having said that, these products may face other challenges, such as the question whether such products can actually be called meat (for which they must be edible parts of animals as indicated above), and to what extent the production technique used must be indicated in accordance with art. 7(1)(a) of, and Annex VI, Part A, point 1 to, the FIC Regulation. We will keep you posted!
Posted: September 20, 2024 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food |
Under the new packaging law, sustainability is no longer a ‘nice to have’ but a ‘need to have’. While companies currently distinguish themselves with recyclable and reusable packaging, this becomes the new normal under the Packaging and Packaging Waste Regulation (‘PPWR’). The PPWR is not yet formally adopted, though informal agreement has been reached at European level on the legislative proposal that was published by the European Commission in November 2022. The new regulation is expected to enter into force later this year.
The PPWR contributes to the transition towards a circular economy and builds on the current Packaging and Packaging Waste Directive, which it will replace. The first major difference can be seen in the mechanism of the law. It is no longer a directive that imposes obligations on Member States, but directly addresses economic operators such as food businesses. This includes shops and catering establishments offering refill and reuse items. The PPWR is a comprehensive piece of legislation. In this blogpost, we list three sustainability requirements under the PPWR that food businesses will face.
(1) Recyclability
One of the first sustainability requirements mentioned in the PPWR is recyclability. This goal must be achieved in two steps. From 2030 onwards, packaging must be designed to be recyclable. A list of criteria showing when this requirement is met is yet to follow. This may mean, for example, that certain hazardous substances that affect the recycling process and make the recycled material unsafe are no longer allowed in packaging. Five years later, from 2035, packaging should actually be recycled in practice. The abovementioned entry deadlines will shift in case the European Commission does not publish on time its clarifying rules as required under the regulation.
For now, food packaging intended for vulnerable groups (think about persons using medical food and baby food), among others, is exempted. For innovative packaging that cannot meet the recyclability requirements taking into account current collection and recycling methods, a temporary five-year exemption can be applied for at national level.
(2) Percentage of recycled material
Plastic packaging still contains relatively little recycled material. In view of the European circularity targets, minimum percentages are now set for this. Taking into account the limited recycling possibilities and food safety, a lower minimum percentage will apply to food packaging than to non-food packaging (namely 30% and 10% from 2030 for PET and non-PET food packaging respectively, compared to 35% from 2030 for non-food packaging). With regard to single-use plastic beverage bottles, the PPWR will replace the minimum percentage of recycled material set for such in the Single Use Plastics Directive as from 2030 (30% by that year). From 2040, the minimum percentages will be further increased for all categories.
How the percentage of recycled plastic in packaging shall be calculated exactly is still to be defined. In any case, exceptions also apply to this sustainability requirement, especially where food safety may be at stake. Packaging with a plastic part representing less than 5% of the total weight of the whole packaging unit is also exempted from the mandatory minimum percentage.
 (3) Compostable
(3) Compostable
Three years after the PPWR comes into force, certain packaging may only be offered in compostable form. In contrast, other packaging may explicitly not be marketed as such, unless such packaging was subject to national compostability requirements before the date of application of the PPWR. The compostability requirement will initially apply to sticky labels attached to fruit and vegetables, and to packaging like permeable tea bags and coffee pads. Aluminum coffee capsules are thus not covered. Non-permeable coffee capsules made of other material may be accepted for composting at national level.
‘Compostable’ under the PPWR means that the packaging complies with the European standard for industrial composting (13432). However, this standard does not match real life industrial composting and will therefore be updated. Member States may as well require that the packaging complies with the standard for home composting. Needless to say, this is a tougher standard. For the time being, the Netherlands has not indicated its intention to make use of this policy freedom.
Chain responsibility
The responsibility to meet the above sustainability requirements lies primarily with manufacturers. These are the (legal) persons who manufacture packaging or a packaged product, or who have such designed or manufactured under their own name or trademark (save for some exceptions). A food company that manufactures (itself) or has manufactured (by a third party) packaged food products is therefore a manufacturer under the PPWR. Manufacturers will have to carry out a conformity assessment. In doing so, they guarantee and declare under their own responsibility that the packaging complies with the relevant provisions of the PPWR. As part of this, the manufacturer shall draw up a dossier demonstrating compliance.
Food companies importing packaging or packaged products from third countries (importers) will have the responsibility to check whether such packaging complies with the Union rules laid down in the PPWR. Should there be a suspicion that the packaging does not comply with the law, this must be corrected. Importers must in such case also inform the competent authority. To avoid misunderstandings and conflicting interests, it is recommended that food companies make contractual agreements on this with their suppliers and/or customers. This also applies to food companies that further market packaging or packaged products on the Union market (distributors). Distributors should also actively ensure that the packaging meets the labelling requirements under the PWWR. This includes facilitating the correct disposal route and combating ‘greenwashing’.
 PPWR: Dynamic legislation
PPWR: Dynamic legislation
The PPWR is a dynamic legislative document. Several criteria still need to be fleshed out in secondary legislation and the PPWR leaves a lot of room for evaluation, additions and deletions (the latter especially with regard to the exemptions it currently contains). However, sitting still and waiting for more clarity is not recommended since developments are moving fast. Our advice: take stock of the current situation within your company, start talking to your suppliers and customers, and check whether current contracts need to be adjusted. And above all, evaluate which sustainability improvements can be made. Because one thing is certain: the EU needs to step up its efforts to become climate neutral by 2050.
A Dutch version of this blogpost is available at VMT.nl.
All images are by freepik.
Posted: September 6, 2024 | Author: Karin Verzijden | Filed under: Food |
 On 5 September 2024, Advocate General (AG) Capeta rendered her opinion in the case initiated by Protéines France, Union Végétarienne, Beyond Meat and others against the French state, disputing a French national Decree limiting the use of meaty names for meat replacements. This Decree dates back to 2022, implementing a specific article of the French Consumer Code. According to this article, the names used to designate foods of animal origin cannot be used to describe, market or promote foods containing vegetable proteins.
On 5 September 2024, Advocate General (AG) Capeta rendered her opinion in the case initiated by Protéines France, Union Végétarienne, Beyond Meat and others against the French state, disputing a French national Decree limiting the use of meaty names for meat replacements. This Decree dates back to 2022, implementing a specific article of the French Consumer Code. According to this article, the names used to designate foods of animal origin cannot be used to describe, market or promote foods containing vegetable proteins.
Two French Decrees
This Decree was immediately under fire by the same parties mentioned above before the French Council of State. According to them, the Decree was unlawful since, roughly speaking, the subject matter was fully harmonized at EU level by the Food Information to Consumers Regulation (FIC Regulation). The French Council of State then stayed the proceedings to ask four explanatory questions to the European Court of Justice (ECJ). Meanwhile, France repealed the 2022 Decree replacing it by a 2024 Decree, essentially similar to the previous one. The question then arose if the request for a preliminary ruling became devoid of purpose. The good news is that according to the AG this is not the case, essentially because the Council of State had informed the ECJ that the interpretation sought remained necessary to enable it to rule on the dispute in the mean proceedings.
System of 2024 Decree
The system of the 2024 Decree is twofold. First, it establishes a list of terms of the use of which is prohibited for the designation of foods containing vegetable proteins (examples: entrecôte, steak, jambon). Second, it authorizes the use of certain terms for the designation of foods of animal origin containing vegetable proteins, provided they do not exceed a certain proportion (examples: cordon bleu (maximum 3,5 % vegetable protein), merguez (maximum 2 % vegetable protein of which 1 % should consist of herbes), terrine de campagne (maximum 5 % of vegetable protein). Products legally manufactured or marketed in another Member State of the European Union or in a third country are not subject to the requirements of this Decree. French companies are however subject to a fine of € 7.500 if they act in violation thereof.
International context
These days, France is not the only Member State where naming issues of meat replacements arise. In fact, in several EU countries such as Italy, Poland and Romania, similar rules as in France have been adopted. Also outside the EU like in the US, in South-Africa and in Switzerland, similar legislative initiatives took place. At the same time, in countries like the Netherlands and in Germany, measures have been adopted expressly allowing the use of meaty names for non-meat products, specifically aiming to prevent any misleading of consumers. For example, in the Netherlands a product can be legally sold under the designation of “vegaschnitzel” (vegetarian Schnitzel) or “vegetarisch krabsalade” (vegetarian crab salad). So, it is about time to have a decision on this topic at European level. Should we be happy with the opinion of AG Cadeta? This most likely depends which side you are on. If you are a manufacturer of conventional meat products, this opinion will most likely meet your approval. If you are a food innovator, proposing alternatives to conventional meat, you will most likely be disappointed. This is why.
AG does not consider use of substitute products harmonized at EU level
The basic question in this debate is whether European legislation has specifically harmonized the naming of substitute products. According to the AG, this is not the case. As a consequence, this leaves room for Member States for establishing legal names, reserving those names for particular foods. Legal names should be distinguished from customary or descriptive names according to their intended effect. If the effect of national rules is that certain names are reserved for certain types of products, then they qualify as legal names. By adopting national measures prohibiting the use of certain customary and descriptive names, including when they are accompanied by additional indications that the product at issue contains plant-based, instead of meat-based, proteins, a Member State turns those customary and descriptive names into legal names. This is precisely the effect of the 2024 Decree and Member States are entitled to do so. The AG goes on to reason that the FIC Regulation does not preclude Member States from adopting national measures according to which meat replacement products can only have a maximum of vegetable proteins when using meaty names. Relevant here is that a distinction is made between domestic production and production abroad. In fact, the 2024 Decree stipulates that its rules do not apply to any imported products. The AG therefore considers the France Decree a purely internal matter. Finally, national administrative penalties sanctioning this regime are not counter to the FIC Regulation.
FIC Regulation does provide for harmonization of replacement products
I do not dispute that Member States can establish legal names at national level; this follows indeed from the FIC Regulation. This may be suitable in the context that food is highly cultural – certain dishes are found in one Member State and not in the other. This is the cultural wealth of Europe, where you only need to travel a few hundred kilometers to find entirely different landscapes, languages and cultural habits. I have however troubles to digest the conclusion that because of the fact that Member States can establish legal names for their specific food products, the subject matter would not be specifically harmonized at EU level. In fact, the FIC Regulation specifically addresses the topic of replacement products in its Annex VI. According to the AG, the rule laid down therein covers the use of meaty names for plant-based substitute products. From the Tofutown decision she however draws the learning that the Annex VI rule only applies if the meaty name is not a legal name. The difference between that case and the present one is that the legal names prohibiting the use of dairy names for dairy replacement products were embodied in the COM Regulation. This is specific harmonization at EU level indeed. In my view, it is questionable if legal names at national level that were newly established for a specific goal (see below) should be attributed the same weight as legal names that have been around for long at EU level, also taking into account the consequences thereof. This is all the more so, now that legal names are usually not prohibited terms as in the 2024 Decree, but positively phrased (example: “The beef and veal sector shall cover the products in the following table: live animals of the domestic bovine species… meats of bovine animals… fats of bovine animals….” – see COM Regulation Annex I, Part XV).
Consequences if ECJ adopts opinion AG Cadeta
If the ECJ embraces this opinion in its decision, there is a good chance that the 2024 Decree will stay in effect and will be enforced in France. Therefore, France national manufacturers of meat substitutes will be very much limited in the designation of their products. As such, they will be put at disadvantage in comparison to manufacturers in countries without such limitative legislation. Furthermore, other Member States having similar legislation in place most likely will feel confirmed in their policy and also enforce these provisions in their home turf. Companies formulating innovative food products thereby get the short end of the bargain on an EU wide basis. And this is not how the internal market was meant.
Two basic principles of internal market under threat
Two starting principles of the internal market are the free circulation of goods and a high level of consumer protection. Allegedly, the rationale behind the French Decrees was consumer protection. The AG explicitly addresses this topic. She states that it does not matter if the French authorities intended to protect consumers or the meat industry, or whether the reason behind such rules is the protection of national gastronomical heritage. At first sight, such a neutral approach could possibly make sense. At second sight, I tend to disagree. In the first place, because experience in the Netherlands has shown that consumers are not misled if meaty names are used for meat replacements. While the Netherlands may be considered very progressive in this regard, it was already established by ProVeg research in 2022 that plant-based labels do not confuse consumers. Secondly, restrictive measures are not needed to protect a certain industry or cultural heritage, since substitute products are not meant to entirely replace conventional meat products. Instead, they offer additional choice to the consumer, who decides to eat less or no meat. This should by no means be a threat to the meat industry, but an increase of food options for consumers. Exactly the purpose of the internal market.
For the reasons set out above, I sincerely hope that the ECJ will not adopt the current opinion of AG Capeta.
Credits image: Vegconomist, 11 April 2024
Posted: August 27, 2024 | Author: Karin Verzijden | Filed under: Authors, Food, novel food |
UK High Court quashes FSA decision on novelty of monk fruit decoctions
Introduction
In the EU, food business operators (FBOs) have the responsibility to ensure that the food they are marketing is not unsafe. Most of the food products do not require prior market authorization, novel foods being one of the exceptions to the rule. For novel foods market authorization is granted based on an extensive safety evaluation by EFSA. If an FBO is unsure if its product / ingredient falls within the scope of the EU Novel Food Regulation, it can submit a voluntary consultation to a Member State, where the FBO intends to first market its product. The Member State competent authority will review the data submitted to establish if a history of use before 1997 can be established. In the affirmative, the product will not be considered novel. Examples of products not considered novel include pea protein concentrate and the Chinese pepper Capsicum chinense.
 This blogpost covers the 19 March 2024 decision of the UK High Court on the novelty of a monk fruit decoction evaluated during a national consultation. This procedure was initiated by Guilin GFS Monk Fruit Corporation (Guilin GFS) against a joint decision of the UK Food Standards Agency (FSA) and the Food Standards Scotland (FSS). Guilin GFS is a world leader producer and manufacturer of monk fruit decoctions, having a 50 % global market share. Monk fruit is a small sub-tropical melon originating from China. Monk fruit decoctions can be applied to a wide range of foods and drinks as a sweetener and is popular for its low-calorie profile.
This blogpost covers the 19 March 2024 decision of the UK High Court on the novelty of a monk fruit decoction evaluated during a national consultation. This procedure was initiated by Guilin GFS Monk Fruit Corporation (Guilin GFS) against a joint decision of the UK Food Standards Agency (FSA) and the Food Standards Scotland (FSS). Guilin GFS is a world leader producer and manufacturer of monk fruit decoctions, having a 50 % global market share. Monk fruit is a small sub-tropical melon originating from China. Monk fruit decoctions can be applied to a wide range of foods and drinks as a sweetener and is popular for its low-calorie profile.
Figure 1 monk fruit image taken from the movie shown at www.monkfruitcorp.com
Applicable test and EC Guidance
After Brexit, the United Kingdom has maintained the EU Novel Food Regulation. Therefore, this decision is also of relevance for EU Member States. Under this applicable framework, the relevant test is whether monk fruit decoctions were used for human consumption to a significant degree in the EU or in the UK prior to 1997 (so-called history of consumption or HoC test). To adduce proof of a HoC, guidance can be taken from the Commission Guidance on Consumption to a Significant Degree dating back to 1997 (EC Guidance). In this context, it is important to note that the EC Guidance itself recognizes the difficulties of proof of significant use by the passage of time. It explicitly states that the examples of use are by no means exhaustive.
Evidence adduced
To meet the test on a HoC, Guilin GFS had adduced substantive evidence, including the following.
- Certificates of origin re. the export of processed foods monk fruits from mainland China to the UK during the period of 1998 – 2000;
- Evidence from a qualitative study from 2018 comprising face to face interviews with 71 participants in the UK and the EU demonstrating that processed foods containing monk fruits were sold in the EU / UK prior to 1997;
- Evidence from over 1.000 questionnaires as part of a quantitative population sample study from 2020 in the UK amongst people from Chinese descent re. their purchases of processed monk fruit;
- A survey in UK / EU supermarkets re. the types of monk fruit products sold before 1998;
- Signed declarations from restaurant owners, FBOs and the London Chinatown Chinese Association attesting the sale and / or consumption of monk fruit decoctions in the UK / EU prior to 1997.
FSA and FFS decision and rationale
On 8 September 2022, FSA and FFS rendered the decision that Guilin GFS did not meet the HoC test (the Decision). FSA and FFS considered the evidence too small in samples and none of this evidence hit the box of “Very Good Evidence”. This is the type of evidence referred to in Table 3 to the EC Guidance reproduced below. The table at stake contains examples of evidence that might be adduced to meet the HoC test, such as invoices detailing the sale of the food product at stake, including evidence of large quantities of the sale in the EU (“Very Good Evidence”), mere invoices detailing the sale of the food at stake (“Good Evidence”) and magazine articles (“Supporting Evidence”). The main reason why FSA and FFS considered the evidence not up to standards was because invoices demonstrating sales of monk fruit decoctions prior to1997 were missing. Also, the FSA considered that personal testimonies were inherently incapable of demonstrating a significant HoC without verification of an independent source.
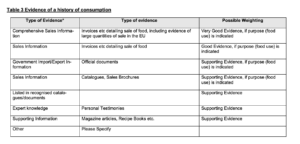
Figure 2: Table 3 to the EC Guidance on Consumption to a Significant Degree
Revision procedure and disclosure of FSA decision making process
Guilin GFS did not agree and initiated a revision procedure before the UK High Court on 2 December 2022, for which the hearing took place on 29 February 2024. The beauty of this procedure is that it provides full disclosure on all relevant documentation leading up to the Decision, including internal FSA correspondence conducted by its Novel Food policy advisors, as well as input from the FSA Social Sciences Team. This is an independent expert team, providing strategic advice to FSA. Contrary to the Novel Food FSA policy advisors, the Social Sciences Team considered the qualitative and the quantitative studies of Guilin to be reliable and robust. The Social Sciences Team did have a few verification requests to FSA’s Novel Food policy officers. However, according to Judge Calver, FSA’s Novel Food policy officers placed these questions out of statistic context to which they relate to validate the conclusion they reached earlier. Also, FSA’s Novel Food policy officers did not ask clarification questions to Guilin GFS when they reached their conclusion that monk fruit concoctions should be considered novel within the UK. Strikingly enough, they even reached this conclusion before the official validation of the dossier.
Debate during revision procedure
Guilin GFS opposed the Decision based on three legal grounds.
(1) It is incorrect that evidentiary requirements of the test for novelty under the Novel Food Regulation could not be met in the absence of pre-1997 sales invoices and data.
(2) It is incorrect that personal testimonies were categorically incapable to demonstrate a significant HoC unless verified by independent source.
(3) It is incorrect that it was necessary to demonstrate that monk fruit was consumed “exclusively for food uses”.
In reply, the Agencies served four witness statements from Novel Food policy advisors to “elucidate the reasons for the Decision.” Justice Calver states that based on the fact that these statements were made 15 months after the Decision, these need to be carefully scrutinized as there is a temptation to bolster and rationalize the Decision under challenge. Evidence that goes beyond elucidation and clarification is not permitted. When evaluating the witness statements, Justice Calver concludes the following.
Ad (1) The Agencies applied Table 3 to the EC Guidance too rigidly. Instead of considering “the whole picture” of evidence submitted by Guilin GFS, the Agencies saw that according to Table 3 the study results submitted by Guilin GFS did not qualify as “Very Good Evidence” or “Good Evidence”. They have then classified them as amounting “only” to “supporting evidence” “as defined in the Guidance”. This sentence makes clear that the Agencies consider anything other than invoices pre-1997 to be an inferior type of evidence as a category, amounting only to supporting evidence, which is not sufficient in itself to demonstrate a significant HoC.
Ad (2) The Agencies erroneously take the view that the evidence submitted by Guilin GFS’s should have been independently verified. There is no such requirement in the law or in the EC Guidance. Furthermore, the studies handed in by Guilin GFS had been qualified as reliable and robust by the Social Sciences Team. Also, with respect to personal testimonies, it is hard to conceive how these should be verified by third parties. As rightfully pointed out by Guilin GFS, such a requirement would frequently render personal testimonies redundant.
As a result, Judge Calver accepts grounds (1) and (2) and he considers the Decision flawed at these points. Moreover, he does not accept the four witness statements, as they try to re-write the reasoning in the Decision, which cannot stand with the wording of that document itself.
Ad (3) Finally, Judge Calver rules that the Agencies misapplied the relevant test when stating in the Decision that it was necessary to demonstrate that monk fruit was consumed “exclusively for food uses. The fact that the evidence submitted by Guilin GFS demonstrated a mixed use of monk fruit, in addition to food uses also including plant based medicinal products and even toothpaste, does not preclude establishing food use prior to 1997 in the EU or in the UK.
As a result, the claim for judicial review succeeds, the Decision is quashed, and the Agencies are ordered to re-consider the Claimant’s application in the light of this judgement. We can now see in the press that the Irish Food Safety Authority is reconsidering the novel food status of monk fruit extract and also the UK’s Food Standards Agency (FSA) has changed its stance regarding these products. The FSA now concludes that monk fruit concoctions are not a novel food, meaning that they can be used in food and beverages marketed in the UK. Reliable sources informed me that in the next weeks the Irish authorities – on behalf of the EU – are expected to issue a similar opinion to the UK.
Does this UK decision also apply for the EU?
In my opinion, this question should be answered positively. Rationale: according to the EC Guidance, an “established history of food use to a significant degree in at least one EU Member State is sufficient to exclude the food from the scope of Regulation (EU) 258/97.” The United Kingdom was an EU Member State during the period to which the evidence collected by Guilin GFS relates. The fact that Brexit occurred in 2020 does not change this. This also follows from the following statement in the EC Guidance: “The deadline 15 May 1997 is applicable to all Member States, irrespective from the date of accession to the EU.” By analogy, the same should apply in case of withdrawal of a Member State from the EU.
What can we learn from this decision?
In the first place, that it is a tough job to collect relevant evidence to establish a history of food use prior to 15 May 1997 in the EU (or the UK), especially since we are more and more moving away from this date. But it is doable. When looking at Guilin GFS, we can see that collecting product information, in combination with qualitative and quantitative data and affidavits can actually help to succeed in establishing such history of food use. Provided of course, you have a skilled lawyer at work to present your case. In the case at hand, the lawyers at work did an excellent job. One of these skilled lawyers is Brian Kelly, with whom I have been working together for more than 10 years now. Kudos to Brian!
Posted: July 25, 2024 | Author: Karin Verzijden | Filed under: Advertising, alternative protein, Authors, Enforcement, Food, Information |
 On 23 July 2024 a Dutch Court ruled in summary relief proceedings that Upfield cannot use the name “roombeter” for a plant-based alternative for butter, as this is in violation of the Regulation establishing a common organization of the markets for agricultural products (“COM Regulation”). You should know that “roombeter” translates in English as a composition of “cream” and “better”, whereas “cream” is a reserved designation under the COM Regulation that can only be used for dairy products. Furthermore, “beter” is close to “boter”, being the Dutch designation for butter.
On 23 July 2024 a Dutch Court ruled in summary relief proceedings that Upfield cannot use the name “roombeter” for a plant-based alternative for butter, as this is in violation of the Regulation establishing a common organization of the markets for agricultural products (“COM Regulation”). You should know that “roombeter” translates in English as a composition of “cream” and “better”, whereas “cream” is a reserved designation under the COM Regulation that can only be used for dairy products. Furthermore, “beter” is close to “boter”, being the Dutch designation for butter.
Facts of the case at hand
In the case at hand, Upfield markets a plant-based alternative for butter under the brand BLUE BAND and the product name ROOMBETER. The packaging of the product furthermore states “100 % plant-based alternative for butter” and “81 % less climate impact than butter”. The packaging itself consists of golden coloured paper that is also used for conventional butter in the Netherlands and it displays a curl of butter as shown below. The Dutch Dairy Association opposed the use of the product name ROOMBETER, as it is considered this a violation of the COM Regulation, as explained below.
Case before Dutch Advertisement Code Committee
Prior to this legal procedure , the Dutch Dairy Association had submitted a complaint regarding this product before the Dutch Advertisement Code Committee. This self-regulatory body ruled on March 21 last that the presentation of the product was misleading, since it could be understood to contain butter. The “e” in “beter” could be confused for an “o”, resulting in “boter”, which is Dutch for butter. And furthermore the golden coloured packaging added to the misleading character of the product. The topic of violation of the COM Regulation was left to civil law proceedings, as it exceeded the competence of the Committee.
Applicable legislation
Article 78.2 of the COM Regulation states that the definitions, designations and sales descriptions provided for in its Annex VII may be used in the Union only for the marketing of a product that conforms to the corresponding requirements laid down in that Annex. Annex VII contains, amongst other things, a product definition for milk and a list of milk products. It furthermore states that these designations may not be used for any other product than milk and milk products. The purpose of this provision is to protect dairy names from being used for non-dairy products.
Tofutown
You may recall that in its Tofutown decision back in 2017, the ECJ formulated a very strict prohibition of the use of diary names for non-dairy products (check out our blog on this case here). As a result of that prohibition, the use of the designation “Tofubutter” for a tofu-based product was in violation of the COM Regulation. As a general rule, the ECJ precluded the term ‘milk’ and the designations reserved by the COM Regulation exclusively for milk products from being used to designate a purely plant based product in marketing or advertising. This even applies if those terms are expanded upon by clarifying or descriptive terms indicating the plant origin of the product at issue
Arguments in favour of ROOMBETER
Upfield had argued it did not market its product under the designation ROOMBETER but under the designation BLUE BAND ROOMBETER. The brand BLUE BAND has been used for more than 100 years for margarine, so it is obvious for the consumer this is a plant-based product This is even strengthened by the Dutch translation of the designations “100 % plant-based alternative for butter” and “81 % less climate impact than butter”. So the name ROOMBETER does not designate, imply or suggest it is about a dairy product.
Court decision
The Court did not eat it. Instead, it very strictly applied the Tofutown doctrine, stating that a reserved designation under the COM Regulation cannot be used for a plant-based product. It went on to explain that if it is prohibited to use the designation “tofubutter” for a plant-based product, as it contains the reserved designation “butter”, for sure it is prohibited to use the designation “roombeter” for a plant-based product, as it contains another reserved designation under the COM Regulation. Also, the element “beter” (“better” in English) can hardly be perceived as a clarifying or descriptive term, as it does not refer (contrary to “tofu”) to a plant-based origin. In fact, its reference to plant-based origins can only be understood by those consumers who know the “skip the cow” ad or who further study the packaging of this product.
Upfield was therefore ordered to stop using the designation ROOMBETER within three months after the date of the legal decision.
Consequences of this decision
Should the conclusion of this decision be that any reference to dairy products should be meticulously avoided when marketing plant-based dairy replacements? This seems a very hard task, as manufacturers of these replacement products will want to indicate how their products can be used. Happily, this is not the case. It is still permitted to mention that your plant-based product is for instance a “yoghurt variation”, as this is perceived as a product explanation rather than a product designation. This is not in violation of the Tofutown doctrine and in line with a 2019 Dutch Supreme Court decision relating to a soy-based product marketed by Alpro. Advertising plant-based dairy alternatives nevertheless remains a delicate balancing act.
Posted: July 23, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Food, Information |
 Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
What is the case about? Advertiser, a food business marketing infant and follow-on formula, published statements on its website about its products such as:
- “Unique combination of natural lactic acid cultures and valuable fibers.”
- “Where organic ends, […] continues: […] guarantees an excellent quality and supersedes the legal requirements concerning organic.”
- “Inspired by nature.”
- “With the first milk that is non mom’s, I want to do all well” (next to a picture of a baby in the arms of a woman).
The question is whether (a) these texts qualify as advertisements, and (b) whether the texts give the impression that the follow-on formula is as good as breastmilk.
What is the legal rule? Consumer-oriented advertising for infant formula is prohibited. Advertisements for follow-on formula are possible, but should not dissuade or discourage breastfeeding. This is laid down in articles 4.1 and 5.2 of the Dutch Advertising Code for Infant Formula, in line with EU Regulation 2016/127. As follows from article 6(6) of aforementioned Regulation, the labelling, presentation and advertising of infant and follow-on formula shall not include terms like ‘humanized’, ‘maternalized’ or ‘adapted’. The Dutch Advertising Code for Infant Formula further lists ‘inspired by breastmilk’, ‘protected effect of breastmilk’, and ‘contains nutrients that are also found in breastmilk’ as examples of prohibited statements to avoid discouragement of breastfeeding.
What was decided? The Board of Appeal upheld the decision of the Advertising Code Committee and ruled that (a) the texts on advertiser’s website are clearly promotional in nature, and that (b) the consumer will understand the references to nature to be references to breastmilk (and not to the products having organic qualities, as was explained by advertiser). As this is in violation of the Dutch Advertising Code for Infant Formula, advertiser is requested to no longer make such advertisements.
 Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Looking into the future, it is interesting to note that the same rules shall in principle apply to cultivated breastmilk made in a lab. Various companies are already working on this concept such as French Nūmi and BIOMILQ in the US. Giants like Danone and FrieslandCampina have announced strategic partnerships with cell-based human milk component start-ups. Companies working on such substitute products obviously want to (and should) explain their products to the public. Statements such as ‘inspired by breastmilk’ and ‘contains nutrients that are also found in breastmilk’ may not seem unreasonable in this context. Currently this is however not allowed. Will the Dutch self-regulatory code (which is more specific in prohibited terms than EU Regulation 2016/127 and the WHO Code) be updated, so that such innovations can be appropriately explained to the public? Stay tuned – we will keep you posted!
The full case can be read here (in Dutch).
Posted: April 24, 2024 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food, Food Supplements, Health claims, Information, Nutrition claims |
 The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
When entering the keyword ‘probiotic’ in the EU Health Claims Register, one is confronted with over 100 rejected health claims. Over the past years, this has led to the question whether the term ‘probiotic’ should be allowed under certain conditions, and has resulted into divergent policies in different EU member states. To protect both the food industry (against unfair competition) and the consumer (against misleading information), these divergent policies are reason for IPA Europe to call for a harmonized framework.
Czech Republic, Northern Ireland and France
Although EU member states generally consider ‘probiotics’ to be an unauthorized health claim under the EU Claims Regulation, some EU member states take a different approach. ‘Probiotics’ is for example considered a nutrition claim in the Czech Republic. Such claim is allowed if the conditions set forth in the EU Claims Regulation are met. This means, amongst others, that the good bacteria in question are present in the food in an amount that, according to generally accepted scientific evidence, causes the claimed beneficial effect. By contrast, in France and Northern Ireland, the term ‘probiotics’ can be used as a general, non-specific health claim that is allowed in combination with the authorized health claim “live cultures in yoghurt or fermented milk improve lactose digestion of the product in individuals who have difficulty digesting lactose”.
Italy
Italy takes the view that the term ‘probiotics’ does not meet the definition of a health claim and therefore falls outside the scope of the EU Claims Regulation. Italy supports this view by EFSA’s conclusion that probiotic colonization in the intestinal flora (without further specification of bacterial species or strains) is insufficient evidence to substantiate a beneficial effect on human health. In other words, no link between probiotics and health can be demonstrated, for which reason ‘probiotics’ cannot be considered a health claim. Aforementioned reasoning is however not a free pass to unconditionally use the term ‘probiotics’ in Italy. Instead, the following conditions must be met if and when using this term: (i) safety for human consumption, (ii) a history of use for the benefit of the intestinal flora, and (iii) presence of the relevant bacteria in the food in live form and in an adequate quantity until end of shelf life.
Denmark and Spain
In Denmark, the term ‘probiotics’ can be used based on a different legal ground, namely as a mandatory category designation under the EU Food Supplements Directive. In France, such is also possible. Indeed, Article 6(3)(a) of the EU Food Supplements Directive requires that the labeling of the food supplement shall bear “the names of the categories of nutrients or substances that characterize the product or an indication of the nature of those nutrients or substances”. In Spain, the claim ‘probiotics’ is allowed thanks to the principle of mutual recognition. Based on this principle, a product lawfully marketed in one EU member state must also be accepted on the market of another EU member state. Since probiotic claims are therefore allowed on the Spanish market for products from other EU member states, banning the term ‘probiotics’ at national level would discriminate against national producers. Therefore, both food products produced within Spain and those produced outside of the country can bear the claim ‘probiotics’.
Netherlands
In the Netherlands – just like in Denmark and France – the term ‘probiotics’ can be used as a category designation for food supplements. This Dutch practice is laid down in the Guideline Document on the EU Claims Regulation by the Dutch Health Advertising Knowledge and Advice Council (in Dutch: Keuringsraad). An earlier version of the Nutrition and Health Claims Manual by the Dutch Food Safety Authority (in Dutch: NVWA Handboek Voedings- en Gezondheidsclaims) also explicitly mentioned this possibility. Such is now longer mentioned in the latest version of the Nutrition and Health Claims Manual, which can be explained by the fact that ‘probiotics’ as a category designation for food supplements is not a nutrition or health claim.
Although claims that further elaborate on the health effect of probiotics (sporadically) occur on the Dutch (online) market, these are not allowed. Whether the expression “increases the good bacteria in the intestinal flora” (without using the term ‘probiotics’) is acceptable in the Netherlands, is yet unclear. Yakult uses this expression to advertise its fermented milk drink. The Dutch Health Advertising Knowledge and Advice Council would not allow this expression in the context of its preventive supervision in the context of food supplements, because this expression in effect creates a link between the food product and health. If the NVWA has however a different opinion on this and does consider aforementioned expression possible, then other food businesses can take advantage of this too. The call for harmonized uses of the term ‘probiotics’ by IPA Europe, about which more below, can possibly contribute to the acceptance of such expression at national level.
”Probiotics’ not a health claim”
As demonstrated above, various EU member states have introduced national rules on the use of the term ‘probiotics’. As a result, IPA Europe believes that the European Commission’s position that ‘probiotics’ implies a health benefit and is therefore a(n unauthorized) health claim no longer holds water. Instead, IPA Europe advocates qualifying the claim ‘contains probiotics’ as a nutrition claim, just like ‘contains vitamins and minerals’ and ‘contains fiber’. Aforementioned substances may have beneficial nutritional properties, but no specific health benefit is claimed. To strengthen its argument that ‘probiotics’ is not a health claim, IPA Europe explains that the term is not sufficiently precise to substantiate the claimed health benefit under reference to an EFSA guidance document published in 2016.
Call for harmonized use of the term ‘probiotics’
According to IPA Europe, ‘probiotics’ is therefore not a health claim and does not require authorization under the EU Claims Regulation. At the same time, it emphasizes the need for clear rules on the use of the claim as this contributes to a fair competitive environment for food businesses and helps consumers to make informed choices. In December 2023, IPA Europe therefore called for a clearly defined framework for the use of the term ‘probiotics’ in the EU. IPA Europe recommends the following four criteria for consistent use thereof:
- characterization of the species level and identification of at strain level;
- the probiotic strain must be safe for the intended use, e.g. based on the QPS list;
- the probiotic status should be scientifically documented; and
- the probiotic strains must be alive in the product and in a sufficient amount up to the end of shelf-life.
Final comment
The EU knows a fragmented regulatory landscape when it comes to the use of the term ‘probiotics’. Despite IPA Europe’s efforts for a harmonized approach throughout the EU, we will need European legislation or a ruling from the European Court of Justice to have the same rules in all EU member states. For now, the term ‘probiotics’ is (fortunately) not completely banned in the Netherlands and neither in quite a few other EU Member States.
This blogpost has also been published in Dutch at VMT.nl.
Posted: December 14, 2023 | Author: Karin Verzijden | Filed under: Food |
 Last month, the conference Regulating the Future of Foods took place in Barcelona, gathered more than hundred professionals active in the fields of precision fermentation and cellular agriculture. The purpose of the conference was to define hurdles and investigate opportunities in the current regulatory framework applicable to this sector. Many interesting presentations took place discussing the global perspective of our future food system and AXON moderated a workshop targeting tastings of cultivated foods, formulating a number of conversation topics. In this blogpost, we share the outcome of the discussions that took place during this workshop.
Last month, the conference Regulating the Future of Foods took place in Barcelona, gathered more than hundred professionals active in the fields of precision fermentation and cellular agriculture. The purpose of the conference was to define hurdles and investigate opportunities in the current regulatory framework applicable to this sector. Many interesting presentations took place discussing the global perspective of our future food system and AXON moderated a workshop targeting tastings of cultivated foods, formulating a number of conversation topics. In this blogpost, we share the outcome of the discussions that took place during this workshop.
Regulatory frameworks for tastings
Tastings of these products already took place, for instance in Israel. So far however only two countries have developed a legal framework for this purpose, notably Singapore and the Netherlands. In the tastings workshop, the procedure and the data requirements for setting up tastings in these countries have been explained, as you can see in the powerpoint inserted below. Furthermore, the following items were discussed.
1. Should the SGP / NL template become the blueprint for tastings or rather do this under the radar?
As follows from the comparison of the regulatory frameworks in Singapore and the Netherlands, these hugely overlap. Therefore, the question arose if these frameworks should become the blueprint for tastings in other countries. Kind Earth.Tech rightfully pointed out that at the beginning of the cultivated meat industry 10 years ago, all tastings held were illegal. They were however important to demonstrate proof of principle and to create appetite for further research. At the current state of the industry, all participants in the workshop however favoured a framework for tastings. Especially for start-ups, a tastings framework is valuable for showing both press and investors what they are up to. Furthermore, the Dutch initiative is useful to convince other EU Member States to develop similar initiatives. Bluu Seafood pointed out that Germany in particular was pretty shy to do so, as it considered tastings not to be in compliance with the EU Novel Food Regulation. Now German start-ups can point to the Dutch framework and request their authorities to take such initiative.
Another reason why tastings might be useful, is that the regulatory process takes a substantial amount of time. In Singapore, predicted timelines for Novel Food approval are between 9 – 12 months, but in reality, these go up. In fact, we have not seen any approvals for cell-based meat products since those for Good Meat and Upside Foods in Q4 2020. In the EU, the theoretic term for Novel Food approvals is about 18 months, but we know from practice it is more realistic to count 2 – 3 years. For the new industries of cellular agriculture, it remains to be seen if this term will apply as well. Demonstrating proof of principle during tastings can be a welcome deliverable before the final go.
Both in Singapore and the Netherlands, tastings should be done in a confined area, not open to the general public. This was understood by all participants in the workshop. At the same time, the concern was expressed that tastings should not become too clinical. They are meant to enjoy food products after all, not to evaluate medicinal products. Provided that a confined area and a selected audience can guaranteed, they can also be set up in a restaurant. Organising tastings during regular opening hours of restaurants will not yet be feasible, as such would fall within the scope of “placing on the market” under article 3.8 of the EU General Food Law Regulation. And placing on the market of Novel Foods requires pre-market approval.
2. For those previously involved in tastings: are they worth the efforts and did they bring you the data you were looking for?
The parameters set in the regulatory tasting frameworks are meant to be high enough to ensure food security but not dissuasive for companies to demonstrate safety prior to obtaining market authorization. Nevertheless, it takes considerable efforts to accommodate these parameters. For instance, producing useful microbiology data requires a minimum quantity of the product to be produced, which at this stage is still very expensive.
Obviously, the tastings themselves require substantial product input, often in combination with various non-cultivated carriers creating a hybrid product. This requires investments which for start-up companies can be challenging.
However, tastings have proved most valuable to collect input from chefs who will be working with cultivated products and to provide proof of principle to investors. In particular, repeating tastings with the same chefs offers the advantage that they can monitor and comment progress made and provide suggestions for further improvement, especially to accommodate the local palette.
2. Is the available guidance from the regulators sufficiently clear to decide how to set up the tasting and establish the safety of your product? Has the regulator been helpful in clarifying any company queries?
Companies that already conducted tastings in Singapore considered the Singapore Food Authority (SFA) to be helpful in not only overseeing this process, but also in facilitating it. Where a first tasting takes quite some work in setting up safety documentation and addressing any potential concerns of the regulator, repeated tastings proved to require much less preparations. This is particularly true if all tastings take place at the same location, as it facilitates the medical contingency plan. This requires the indication how much time it takes to reach a medical centre if any problems occur.
Most importantly, permission for tastings being granted is perceived as a quiet vote of confidence by the regulator, as it seems to have a minimum level of comfort with the safety of the product to be tasted.
3. Do you see any value in tastings to be set up at an EU level as opposed to national level only?
No, not really. It is important to create a product that appeals locally, and this may differ from community to community. Also, it was discussed that tastings organized at a central level could induce companies to rely on the greatest common denominator, i.e. a burger. This would in fact be a pity, as cultivated foods offer so much more opportunities than that. This was for instance demonstrated by Vow Foods who made a crème brûlée with its cultivated quail.
4. Any ideas what could be done to prevent that tastings slow down regulatory approvals?
The representative from SFA explained that the total staff available for the safety assessment of Novel Food AND the evaluation of an exemption for the tasting and sensory evaluation of Novel Foods is four to five persons. It is easy to understand that the more time is spent on the evaluation of tastings, the less time can be spent on the safety assessment of Novel Foods as such.
It was discussed that if tastings go wrong, for instance by creating a health problem, they imply a risk, not only for the product at stake, but also for the sector. On the other hand, tastings also spark enthusiasm, as was demonstrated by Solar Foods who even held tastings after obtaining market approval in Singapore.
So the communis opinion was that tastings should be dosed. Do not overdo it, but carefully weigh the advantage it can bring your company at a particular moment of its lifetime, for example during a funding round.
5. Do tastings qualify as studies under the EU Transparency Regulation, that should be notified to EFSA during an application for authorization of a Novel Food?
The EFSA representative present in Barcelona was quite outspoken regarding this question. If you have any concerns about toxicity or any other possible hazard, you do not want to expose your intended audience for tastings to such hazard. A tasting session is not a study. So no, tastings as such do not have to be notified to EFSA under the Transparency Regulation.
What’s next?
In the Netherlands, the CAN Expert Committee is expected to be nominated early 2024 and local companies are already gearing up for organizing tastings in situ. So soon the Dutch will continue to work on their Dutch dream. Watch that space!
Posted: December 8, 2023 | Author: Jasmin Buijs | Filed under: Food |
 Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
This question stems from proceedings initiated by the German unfair competition association Verband Sozialer Wettbewerb e.V.. We owe many ECJ rulings to this German association, including the famous TofuTown ruling from which it follows that milk designations are reserved for animal dairy products only. We covered the TofuTown case in an earlier blogpost. The current case is against the food supplement company Novel Nutriology, which sells, among other things, an ‘anti-stress’ supplement containing saffron and melon juice extract.
The saffron extract is said to create a more positive mood, according to the seller’s website. This expression is backed by the results of an open study on 50 participants over a 30-day period. Research has shown that the melon juice extract reduces feelings of stress and fatigue, according to the website. Verband Sozialer Wettbewerb e. V. considers these to be unpermitted health claims. It therefore requested Novel Nutriology to stop making these claims, but the supplement company did not listen.
Approved health claims
Health claims are in principle only allowed if they are included in the lists of approved claims under the EU Claims Regulation. This follows from article 10(1) Claims Regulation. References to general, non-specific health benefits such as ‘heart health’ and ‘mental energy’ are allowed if accompanied by a specific, approved claim (article 10(3) Claims Regulation). However, health claims for botanicals are ‘on hold’ and are therefore not included in the lists of approved claims. Therefore, the literal text of Article 10(1) and (3) Claims Regulation cannot be met when making health claims for botanicals.
Claims Regulation nevertheless applicable to botanicals?
To determine whether Novel Nutriology violates the prohibition on making unauthorized health claims, it is essential to know whether Article 10(1) and (3) of the Claims Regulation apply to botanicals. If not, violation of these provisions is out of the question.
As can be seen from the summary of the request for the preliminary ruling, the view is generally taken that references to general health benefits of botanicals must comply with Article 10(3) Claims Regulation. The requirements of this provision are met if the generic health benefit is accompanied by the full, specific health claim that is ‘on hold’. That full ‘on hold’ claim, although not officially authorized, may be used in accordance with the transitional measures in Article 28(5) and (6) Claims Regulation. It would not be compatible with the purpose of Article 10(1) and (3) Claims Regulation to exclude these provisions entirely for botanicals. Such would namely mean that botanical substances may be advertised with non-specific health claims without a scientific assessment of the specific claim supporting them.
Alternative views
Nevertheless, the Bundesgerichtshof finds it unclear whether Article 10(1) and (3) Claims Regulation apply to botanicals. As an argument against applicability, the German court states that the Union legislator would have considered an absolute ban on general health benefits as too broad. Therefore, the Union legislator intended to ban such only if the general claim is not accompanied by an approved, specific health claim.
It is currently however impossible to obtain approval for botanical claims that are ‘on hold’ due to the European Commission’s inaction. By making Article 10(3) Claims Regulation applicable to these substances, the prohibition becomes broader than the Union legislator would have intended. It should therefore be assumed that general health benefits for botanicals are not regulated until the European Commission continues the authorization procedure for ‘on hold’ claims. Based on this alternative view, Article 10(3) of the Claims Regulation should not apply until then.
A second argument raised against the applicability of Article 10(1) and (3) Claims Regulation to botanicals is that the European Commission has not taken action on ‘on hold’ claims for many years. Upholding the applicability of Article 10(1) and (3) Claims Regulation, which cannot be met for ‘on hold’ claims, would lead to a disproportionate restriction of the interests of companies making such claims.
Previous ECJ rulings
 Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Aforementioned report also highlights that the ECJ previously made explicit that Article 28(5) Claims Regulation makes an exception for the use of specific health claims as referred to in Article 13(1)(a) Claims Regulation that have not yet been officially approved. The report therefore concludes that the mentioned transitional regime only applies to the full specific claim and not to general health benefits. General health benefits would therefore not be allowed, although it is recognized that some EU member states do accept general health benefits for botanicals when accompanied by the full ‘on hold’ claim.
Relevance for practice
Health claims for botanicals are currently accepted in the Netherlands if the roadmap of the Dutch agency regulating health products (the Keuringsraad) in cooperation with the NVWA, is met (see here, in Dutch). In brief, this means that (i) the substance and claim are present on the ‘on hold’ list, (ii) potential conditions of use such as a daily dosage are included on the label, (iii) the claim is in line with the wording accepted on KOAG KAG’s ‘indicative list’, and (iv) a disclaimer regarding the ongoing approval procedure is made.
If the ECJ rules that Article 10(1) and (3) Claims Regulation does not apply to botanicals, it is uncertain whether the Keuringsraad roadmap can be upheld. It is however more likely that the ECJ will clarify that botanicals do fall within the scope of the aforementioned provisions. In that case, various outcomes are still possible. In connection with the transitional provisions of Article 28 Claims Regulation, a difference may arise for full ‘on hold’ claims and general health benefits. Such will partly depend on whether the ECJ sticks to the literal text of the transitional provisions (which would prohibit the use of general health benefits), or takes into account current practices (in which case advertising with general health benefits remains allowed under certain conditions). To be continued!
 On 5 September 2024, Advocate General (AG) Capeta rendered her opinion in the case initiated by Protéines France, Union Végétarienne, Beyond Meat and others against the French state, disputing a French national Decree limiting the use of meaty names for meat replacements. This Decree dates back to 2022, implementing a specific article of the French Consumer Code. According to this article, the names used to designate foods of animal origin cannot be used to describe, market or promote foods containing vegetable proteins.
On 5 September 2024, Advocate General (AG) Capeta rendered her opinion in the case initiated by Protéines France, Union Végétarienne, Beyond Meat and others against the French state, disputing a French national Decree limiting the use of meaty names for meat replacements. This Decree dates back to 2022, implementing a specific article of the French Consumer Code. According to this article, the names used to designate foods of animal origin cannot be used to describe, market or promote foods containing vegetable proteins. Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives. Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling. Last month, the conference Regulating the Future of Foods took place in Barcelona, gathered more than hundred professionals active in the fields of precision fermentation and cellular agriculture. The purpose of the conference was to define hurdles and investigate opportunities in the current regulatory framework applicable to this sector. Many interesting presentations took place discussing the global perspective of our future food system and AXON moderated a workshop targeting tastings of cultivated foods, formulating a number of conversation topics. In this blogpost, we share the outcome of the discussions that took place during this workshop.
Last month, the conference Regulating the Future of Foods took place in Barcelona, gathered more than hundred professionals active in the fields of precision fermentation and cellular agriculture. The purpose of the conference was to define hurdles and investigate opportunities in the current regulatory framework applicable to this sector. Many interesting presentations took place discussing the global perspective of our future food system and AXON moderated a workshop targeting tastings of cultivated foods, formulating a number of conversation topics. In this blogpost, we share the outcome of the discussions that took place during this workshop. Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims. Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.










