Posted: August 10, 2015 | Author: Karin Verzijden | Filed under: Authors, Enforcement, Food, Health claims, Information, Nutrition claims |
 The Netherlands Food and Consumer Product Safety Authority (NVWA) has been pretty active this Summer. Following up on previous enforcement reports on nutrition claims, it recently published such enforcement report re. both nutrition and health claims for breakfast cereals. As this report relates to legislation that has been harmonized at an EU-wide level and it provides detailed enforcement information, this is an interesting read – not only for the Netherlands.
The Netherlands Food and Consumer Product Safety Authority (NVWA) has been pretty active this Summer. Following up on previous enforcement reports on nutrition claims, it recently published such enforcement report re. both nutrition and health claims for breakfast cereals. As this report relates to legislation that has been harmonized at an EU-wide level and it provides detailed enforcement information, this is an interesting read – not only for the Netherlands.
Less than 50 % fully compliant
Actually, the report publishes data on the review of claims regarding 126 different breakfast cereals marketed under 24 different brands. These data were compiled during the period between March – October 2014. From this review, It appeared that less than half of those products were fully compliant with the Claims Regulation. The NVWA considers it of the essence that Food Business Operators (FBO’s) offering for sale breakfast cereals fully comply with the Claims Regulation to enable consumers to make informed choices.
Nutrition and health claim framework
Since the entry into force of the Claims Regulation on 1 July 2007, only authorized claims can be used for food products. A claim is a message or representation in any form, that is not mandatory under EU law or national legislation and that states, suggests or implies that a food has certain characteristics. A nutrition claim is a claim that states or implies that a food has particular beneficial nutritional properties in terms of energy and/or nutrients. A health claim is a claim that states or implies there is a relationship between food and health. Amongst the health claims, a distinction is made between general claims, disease risk reduction (DRR) claims and claims relating to the health and development of children. In 2012, the Commission published a list of 222 authorized general claims that is dynamic and has currently evolved into 229 claims. Furthermore, there are currently 14 authorized DRR claims and 11 children’s claims. For both nutrition and health claims, strict conditions of use are applicable. For instance, in order to claim that a product is high in proteins, at least 12 % of the energy delivered by the product should be provided by proteins.
Method of enforcement
Contrary to previous claims enforcement reports that only related to nutrition claims, the NVWA this time also took into account health claims. More concretely, it report relates to pre-packed breakfast cereals that were offered for sale in the Netherlands at both retail and wholesale level. If the products at stake were advertised at websites as well, such information was also subject to enforcement. The products at stake consisted of granola, corn flakes, puffed rice grains, oatmeal and wheat meal, some of them supplemented by nuts, sugar, dried fruits or chocolate. The information reviewed was the name of the product and the nutrition labelling, in as far as related to nutrition and health claims. A warning letter was sent to those FBO’s whose products were not compliant, except when an unauthorized claim was used. In those cases, a fine was imposed under the suspensive condition of full compliance within a grace period. When I contacted the NVWA to know the average amount of such fine, I was informed that my query would be answered within 6 weeks. Apparently, the Summer scheme is still on at NVWA – too bad. But an update will be provided at FoodHealthLegal when more information is available.
Outcome of enforcement
As mentioned above, out of the 126 products that were reviewed, over half of them did not fully comply with the Claims Regulation, mostly because the claims used were pretty vague. Although it is permitted to use a variation on an authorized claim, the essence thereof should be the same as an authorized claim. An example of a claim that was sanctioned by the NVWA is “The cereals present in this product are the basis for a healthy and nutritious breakfast. It contains nutrients that are indispensable for the human body and that are quickly absorbed by the body.” Another reason why products were not compliant was that non-existent nutrition claims were used, such as “does not contain cholesterol” or “ contains many nutrients”. Furthermore, sometimes nutrition claims were made, whereas the strict conditions of use were not met. Although this is not visible from the product label, such is a violation of the Claims Regulation as well. Finally, on many products, the link between a specific nutrient and the health claim used on the labelling was missing. Instead, pretty general, non-specific, claims were made that can be quickly taken in by the consumer, but also easily be misunderstood. Therefore, such general, non-specific claims are only allowed if accompanied by a specific, authorized, health claim. For example, the claim “Crisply granola of brand X forms part of a healthy and nutritious breakfast” cannot be used alone, but it can be used jointly with an authorized claim for iron, like “Iron contributes to normal energy-yielding metabolism”.
Recommendations
The fact that the NVWA nowadays actively enforces health and nutrition claims shows that it considers B2C communication on food product to be an integral part of food safety. From our practise I know changing the packaging of your food products is a lot of hassle, so better get it right as from the start. Here are a few tips to help you along.
- At all times, it should be avoided to made a medical claim with respect to food products. Medical claims are claims directed at the prevention or treatment of a disease. Their use, if allowed at all, is strictly reserved for pharmaceuticals.
- If you consider the authorized claims are not persuasive or sexy enough, choose one of the variations published by the self regulatory body KOAG-KAG.
- Make sure you have data supporting the nutrition facts of your food product, as the burden of proof lies with the FBO when receiving a request pertaining thereto from the NVWA.
- When using general, non-specific claims, use the specific, authorized claim in the same field of vision.
- When in doubt, or if you simply need a sparring partner, consult an expert.
Posted: October 7, 2014 | Author: Karin Verzijden | Filed under: Authors, Food, Health claims, Nutrition claims |
 The use of nutrition and health claims is gaining wider application in the food industry. At the same time companies in the horticultural sector focus their marketing more and more at end users of their products. On the basis of this development, the Dutch Kenniscentrum Plantenstoffen (knowledge institute for plant compounds) initiated an investigation whether and how the vegetable chain can benefit from the use of nutrition claims. This information is relevant for breeders, growers, retailers, processors and consumers of vegetables.
The use of nutrition and health claims is gaining wider application in the food industry. At the same time companies in the horticultural sector focus their marketing more and more at end users of their products. On the basis of this development, the Dutch Kenniscentrum Plantenstoffen (knowledge institute for plant compounds) initiated an investigation whether and how the vegetable chain can benefit from the use of nutrition claims. This information is relevant for breeders, growers, retailers, processors and consumers of vegetables.
Central concepts
The so-called claim legislation (EC Regulation1924/2006 and EC Regulation 116/2010) is applicable to all food and distinguishes between nutrition and health claims. These and the central concept of foodstuff have been defined as follows.
• Foodstuff: any substance or product, whether processed or unprocessed, intended to be consumed by humans. This includes, of course, vegetables.
• Claim: any non obligatory indication that states or implies that a food has particular characteristics, including graphics representations and symbols.
• Nutrition claim means any claim that the impression that a food has particular beneficial nutritional properties with regard to energy, and / or nutrients (“What is in the product?“)
• Health claim means any claim that the impression that there is a relationship between a food and health (“What does the product do?”).
Importance of claims for the horticultural sector: targeted breeding and communication
The Kenniscentrum Plantenstoffen chose to focus its investigation of the importance of nutrition claims for the horticultural sector. The use of health claims generally requires further research and evidence than is required for the use of nutrition claims. The importance of claims for the horticultural sector is twofold. Not only offer claims the opportunity to provide detailed information about specific nutrients in a product. Also the possibility of the use of a nutrition claim can offer an incentive for product improvement. When it is known which claims are relevant in connection with which vegetables and what conditions must be satisfied, is it possible to adjust the marketing and / or the breeding activiteis accordingly. For this purpose two documents were produced.
(1) List of permitted nutrition claims
(2) List of permitted vitamins and minerals associated with specific nutrition claims
List of permitted nutrition claims
Based on the current state of the law, there are a total of 29 permitted nutrition claims, including, for example, “low fat” or “sugar free“. A breeding company will however not directly benefit from these examples. Therefore, a selection of claims has been made that potentially offers added value to the marketing of vegetable breeders, growers or other horticultural companies. On this basis, 13 potential valuable claims remained. For all these claims, the terms of use were specified and also the vegetables for the marketing of which they could be used. An example of such selected claim reads as follows.
| Selected nutrition claim |
Condition for use |
Relevant crops |
| High fibre |
Product should contain at least 6 g fibre per 100g or 3 g fibre per 00 kcal. |
Cauliflower, broccoli, cabbage, beans, Romaine lettuce and celery |
List of permitted vitamins and minerals
A special group of nutrition claims are the claims that read “source of [name of vitamin / mineral]” and “rich in [name of vitamin / mineral]”, which may be used solely in conjunction with permitted vitamins and minerals. The applicable legislation (currently EC Directive 90/496 and as per December 13, 2014 EC Regulation 1169/2011) identifies 27 permitted vitamins and minerals. 16 of them have been identified as potentially interesting in connection with plants and flowers. Subsequently, it has been established which claim can be used in connection with which vegetable, both per 100g and per portion. An example is as follows.
| Selected vitamine |
Nutrition claim: “high on [selected vitamin] |
|
Per 100 g |
Per portion |
| Vitamine A |
Carrots (raw and cooked), Cantaloupe melon, spinach, kale, chard, celery and chili |
Carrots (raw and cooked), Cantaloupe melon, spinach, kale, chard, celery and chili |
Explanation
In order to make a selection of crops in the list of nutrition claims, the following actions were taken per ingredient: (i) identification of crops rich in fiber (in particular example stated above) in two independent sources, (ii) selection of relevant Dutch horticultural crops, (iii) identification of the quantity of fiber per 100 g and per portion on the basis of two independent sources, and (iv) selection of crops on the basis of the so-called reference intake. For the selection of crops on the list of vitamins and minerals, an identical selection process was applied.
Qualifications
Despite the care applied to the selection of crops included in the list of nutrition claims, this selection is of course not set in stone. The reason is that the amount of nutrients (such as vitamins and minerals) is dependent on the specific cultivar per crop. Furthermore, growing conditions such as sun, soil, water and temperature affects the amount of nutrients present in crops. Finally, in the literature consulted regarding nutrients (including vitamins and minerals) intra-and intercontinental differences were found in the same crops. In addition, differences may exist within the same crop per portion: the weight of one tomato does not necessarily equal any other tomato.
Useful guidance
Despite the above qualifications, the lists of authorized nutrion claims and vitamins & minerals can be considered as a good indication for which nutrition claims could be used in combination with which vegetable. These lists will be published shortly at this website and they can serve as a new perspective for the Dutch horticultural sector for their marketing and communication of their crops. With respect to vitamins and minerals are also a large number of health claims is available. The Kenniscentrum Plantenstoffen will look into this in the near future.
Health and nutrition claims targeted at food and medical products
For further information on the use of health claims targeted at different products, reference is made to our previous post on this blog. The slides of our seminar on medical and functional foods can be found here.
Posted: August 15, 2014 | Author: Sofie van der Meulen | Filed under: event, Food, Food Supplements, Health claims, Information, novel food, Nutrition claims |
 On 24 September 2014 our firm organizes a seminar addressing the developments in Functional and Medical Foods. Where? Next door to our office in Pakhuis de Zwijger.
On 24 September 2014 our firm organizes a seminar addressing the developments in Functional and Medical Foods. Where? Next door to our office in Pakhuis de Zwijger.
Functional foods
The Institute of Medicine has designated functional food as “any food or food ingredient that provides a health benefit beyond its nutritional benefit”. Functional foods comprise food supplements, probiotics and other foods having a beneficial effect on human health, which is often advertised by health or nutrition claims as using those claims in connection with this type of foods is very attractive from a marketing point of view.
Medical foods
Functional foods are just one step away from medical foods, catering for particular nutritional needs that regular foods do not supply. Contrary to functional foods, medical foods are highly regulated. Furthermore, both types of food are subject to specific legislation regarding health and nutrition claims as well as regarding food information to be provided to consumers.
The regulatory requirements for health and nutrition claims offer a chance and a challenge. Also, as of 13 December 2014, all food business operators have to comply with very strict, new information requirements on the basis of the Regulation on Food Information to Consumers. To what extent does this regulation help the final consumer to make healthy choices and how does this impact the entrepeneur? Our seminar will address these issues and provides practical guidance on solutions. Speakers are: Bernd Mussler (Innovation Project Manager at DSM), Ruud Albers (CEO at Nutrileads) and us (Karin Verzijden and Sofie van der Meulen).

A few spots left!
We still have a few spots left! Attendance is free and you are welcome to bring along your colleagues. More info? Check the invitation on our website. For registration, please send an e-mail to info@axonlawyers.com with your name, the names of colleagues you would like to register and contact information. Questions? Contact our office manager Marjon Kuijs at marjon.kuijs@axonlawyers.com or +31 (0) 88 650 6500.
Looking forward to see you there!
Missed our seminar? Download our slides!
The slides of our seminar on medical and functional foods can be found here and also via our SlideShare account.
Posted: June 30, 2014 | Author: Karin Verzijden | Filed under: Authors, Food, Health claims, Information, Nutrition claims |
 From 21 – 24 June, the yearly conference of the International Food Technologists (IFT) took place in New Orleans, Louisiana. During this conference, over 16.000 food science and technology professionals discussed the most recent product, ingredient and technology developments, as well as the potential business impact and regulatory framework. Below, you will find a selection of topics discussed. It will also be commented how these impact food business operators in the EU.
From 21 – 24 June, the yearly conference of the International Food Technologists (IFT) took place in New Orleans, Louisiana. During this conference, over 16.000 food science and technology professionals discussed the most recent product, ingredient and technology developments, as well as the potential business impact and regulatory framework. Below, you will find a selection of topics discussed. It will also be commented how these impact food business operators in the EU.
Functional foods
So far, no proper legal definition exists of functional foods. Since 1984 however, the Institute of Medicine has designated as functional food “any food or food ingredient that provides a health benefit beyond its nutritional benefit”, which I consider a clear working title. In the functional food market, functional beverages are the fastest-growing sector. In the IFT-session “The evoluation of functional beverages” advances in ingredients were discussed, such as new vitamin forms, dietary fibers and certain omega-3 fatty acids. Also, trends towards more natural forms of coloration were discussed, for instance from lycopene and beta-carotene, which also increase antioxidant properties. Obviously, using health claims in connection with these functional foods is very attractive from a marketing point of view. However, this remains a strictly regulated area in the USA, where different rules apply to “classical” health claims, structure function claims and qualified health claims. As discussed in our posts of 8 May 2014 and 2 November 2013, this is not any different in the EU.
Health benefits of prebiotics
In IFT-session Presenters Detail Gut Health Research some of the latest research was shared on the ways in which prebiotics affect the gut microbiota. Prebiotics are substances that are selectively fermented by gut microbes, thereby delivering health benefits to the host. It was reported that during the last 20 years, research has shown that prebiotic ingredients stimulate the growth of beneficial gut bacteria. Furthermore, evidence has been obtained suggesting that diet can contribute health benefits by changing the composition of the gut microbiota. It was pointed out during this presentation, however, that regulatory bodies like the EFSA have refused to permit health claims for prebiotics. Although this may be true in general, it is not entirely correct for lactulose, regarding which EFSA’s NDA panel acknowledged the beneficial effect consisting of a reduction of transit time.
Allergens
From the session Allergens-Free Food Formulation, it became obvious that allergies (being defined as an abnormal response to a food triggered by the body’s immune system) constitute a prominent item on the agenda of food business operators for the years to come. Currently, millions of people have food allergies and that total is growing. Presenters during this session referred to the statistics from the Centers for Disease Control, according to which food allergies among children surged by 50% between 1997 and 2011. In order to respond appropriately, food manufacturers not only have to formule allergen-free foods, but also create an allergen-free manufacturing environment. Also, all of this should be effectively reflected in the product information, in order to meet the applicable labeling requirements. As reported already in our post of 17 September 2013, with the entry into force of the Food Information Regulation by the end of this year, specific measures apply regarding 14 named allergens, both for pre-packed and non pre-packed food.
Product qualification: food or pharmaceutical?
In the session Food Clinical Trials and IND-applications, it was explained when clinical studies for foods would require the filing of an Investigational New Drug (IND) application. This question was answered based on a recent guidance document (Guidance for Clinical Investigators) issued by 3 organisations involved in the evaluation of drugs, biologicals and food and of course on the basis of the criteria contained in the Federal Food, Drug & Cosmetic Act (FFD&C Act). It was explained that if the intent of the clinical study is to investigate a physiological effect of the food on the structure or function of the body beyond the provision of taste, aroma, or nutritive value, then the study will require the filing of an IND application. Furthermore, guidance can be found in FFD&C Act, according to which a food qualifies as a drug if it is intended for use in diagnosis, cure, mitigation, treatment or prevention of disease and if it is intended to affect the structure or any function of the body inter alia. In the EU, we regularly see legal decisions dealing with similar qualification issues based the European Drug Directive. Clearly, this is a topic in the centre of interest on both sides of the Atlantic. It is therefore expected that US guidance on this topic may be of interest for its European counterparts as well.
Further topics @ IFT and subsequent updates @ FHL
Other topics discussed at IFT were overweight and obesity, perception of food in media and GMO’s in the food industry. At FoodHealthLegal, we continue to follow all these topics, and in particular their legal and regulatory implications, with interest and we will provide you with further updates shortly. Please feel free also to join Axon’s life sciences seminar on medical and functional food, which will be held on 24 September 2014.
Posted: April 18, 2014 | Author: Karin Verzijden | Filed under: Food, Food Supplements, Health claims, Information, Nutrition claims, Uncategorized |
The role of national customs and self-regulatory bodies
Introduction
Recently, the Rotterdam District Court decided a dispute between Omega Pharma and Procter & Gamble on allegedly misleading advertising of food supplements. The product in dispute was Vibovit, a multi vitamins product for mothers and young children marketed by Procter & Gamble (P&G) since January 2014. Although well-known for its consumer products such as detergents and toothbrushes, Procter & Gamble is a new player in the field of food supplements. Omega Pharma is a company focusing on OTC health and care products including food supplements, for instance Davitamon. It holds a market share of 61 % in volume and 65 % in value in this field. Omega Pharma clearly perceives Vibovit as a competing product for Davitamon. No doubt, clear scrutiny of the new competitor in the field of food supplements was the start of this case.
Claims made by Omega Pharma
In a nutshell, Omega Pharma accuses P&G of committing misleading advertising and unfair commercial practises regarding its Vibofit product and also of violation of legislation on health claim, food supplements and labeling. More concretely, Omega Pharma inter alia opposed (1) the claim that Vibofit did not contain any preserving agents, whereas it appeared from the list of ingredients that the product contained potassium sorbate. (2) Furthermore, Omega Pharma complained that the packaging of Vibofit stated it contained only natural colouring agents, as it did not agree that the colouring agents titanium dioxide and carbon dioxide qualified as such. (3) Also, Omega Pharma considered the claim “+Omega 3” mentioned on the Vibofit packaging was made in violation of the Claims Regulation.
P&G’s preliminary defence
As a first preliminary defence, P&G had argued that Octapharma’s claim could not be received by the Civil Court, as Octapharma should have brought this claim before the Dutch self-regulatory body that advises on advertisements for health products (KOAG-KAG). This defence was rejected, as this self-regulatory body is only competent to hear disputes on health claims and not regarding any other claims in the field of food or advertising law. As a second preliminary defence, P&G had advanced that Omega Pharma’s claim actually related to unfair B2C commercial practices and that Omega Pharma could not invoke these rules (meaning their national implementation) against its competitor. This defence was also dismissed, as the Court considered that Omega Pharma was entiteld to invoke the rules on unfair commercial practises, being a lex specialis on the general law of the torts, against P&G.
(1) Evaluation of claim re. preserving agent potassium sorbate
Although the parties agree that potassium sorbate can be used as preserving agent, P&G argues this is not the case at hand. Instead, it uses this compound as a processing agent for the preparation of the yellow colour of the Vibofit gummies. As a result of the carry-over principle, a mimimum quantity of potassium sorbate is present in the final product. However, since this quantity is only 0,00005 %, which is 2.000 times too weak to be able to function as s preserving agent, the Court accepts this defence. As a consequence, the claim “free of preserving agents” is not considered misleading.
(2) Evaluation of claim re. colouring agents titanium dioxide and carbon dioxide
Omega pharma opposes the claims “100 % natural colouring agents” and “no artificial colouring agents”, as it considers that both titanium dioxide and carbon dioxide qualify as synthetic instead of natural colouring agents. Since the Food Additives Regulation does not make a distinction between natural and synthetic colouring agents, P&G relies on evidence from two national authorities to refute Omega Pharma’s claim. The first piece of evidence is a list of additives issued by the Dutch Food Safety Authority (Nederlandse Voedsel en Waren Autoriteit – NVWA) based on their qualification by the Dutch Nutrition Center (Voedingscentrum). This authority qualifies titanium dioxide (E171) and carbon dioxide (E172) as “natural”, as opposed to “synthetic” and “of natural origin, chemically processed”. The second piece of evidence is an NVWA fact sheet from which is follows that the colouring agents E171 and E172 are not considered synthetic. A consumer survey initiated by Omega Pharma investigating the misleading character of the claims used by P&G was considered not relevant, inter alia because it did not outweigh the opinion of the two Dutch national authorities and it was not considered completely neutral. As a consequence, P&G’s claims were not considered misleading.
(3) Evaluation of health claim +Omega 3
The notion + Omega 3 relates to the nutrition claim “source of Omega-3 fatty acids”. According the Health Claim Regulation, this claim is only allowed where the minimum quantities of 0,3 g ALA or 0,40 mg of the sum of EPA and DHA are met. One Vibofit gummy contains 2,5 mg Omega 3. As the packaging does not differ amongst ALA, EPA or DHA, it is not clear if the applicable standards are met. P&G however argues that it received prior approval from the self-regulatory body KOAG/KAG regarding its Omega 3-claims. Such approval has such authority that in those cases the Dutch Food Safety Authority usually does not apply any fines. In this particular case however, KOAG/KAG changed its policy after said prior approval was granted. According to the new policy, ingredients claims will only be allowed in as far as no existing health or nutrition claim is in place. Although P&G was not happy with this change, it nevertheless removed the Omega 3 claims from the Vibovit packaging and showed the galley proofs during the hearing. As a consequence, the Court considered that Omega Pharma no longer has sufficient interest re. its claim directed at Omega 3.
Conclusion
From the correspondence exchanged between the parties and reproduced in the judgement, it appears that P&G did not simply dismiss Omega Pharma’s claim but had meticulously prepared this case. Where it estimated that its claims would not hold, it had decided to move and to do so quickly. This is of course a very strategic approach, which quite often is successful to avoid litigation. However Omega Pharma simply decided to go after its new competitor. What is most striking in this case from an EU perspective, is the role of national authorities and national customs formulated by self-regulatory bodies. The prior approval from KOAG/KAG with respect to an ingredients claim for the food supplement Vibovit carried an enormous weight in the present dispute. Also, interpretations of national authorities of European food additives standards proved to be a decisive factor in this case. Therefore, when preparing a case of misleading advertising involving labelling and health and nutrition claims, carefully consider where to initiate it in view of those local customs.
Posted: August 14, 2013 | Author: Karin Verzijden | Filed under: Food, Information, Nutrition claims |
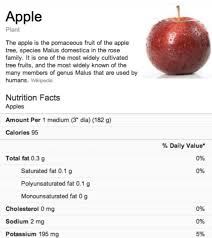 Food information services by Google
Food information services by Google
Recently, Google added nutritional information into its search functionality relating to more than 1,000 food items ranking from fruits to vegetables and from meats to complete meals. The new functionality is a part of Google’s Knowledge Graph that was launched in May 2012 in the US. Knowledge Graph is a database interconnecting various search results in order to enhance understanding. By offering information in this way, Google aims developing its information engine into a knowledge engine. As to food information, Google wants to help its users to make healthier choices – so it says. For examples how Google’s food information service will be operated, reference is made to its blogspot.
Food information in the EU
In the European Union, food information is considered to be of the essence as well. To that end, rules on food information were revamped under Regulation 1169/2011 (“the Regulation”) on the provision of food information to consumers . This Regulation will enter into force on 13 December 2014 and will bring about a great number of changes in the food information landscape in Europe. On the one hand, the Regulation aims to ensure a high level of protection of consumers’ health and interests by enabling them in making informed choices. On the other hand, the Regulation aims to realise free movement of legally produced and marketed food. But how is this going to be achieved?
Fair information practices
Firstly, food information shall not be misleading (a) as to the characteristics of the food, (b) by attributing to the food effects or properties it does not possess, (c) by suggesting that the food possesses special characteristics when in fact all similar food possess such characteristics, (d) by suggesting the presence of a particular food or an ingredient, while in fact substitution with another food or ingredient occurred. It should be stressed that food information comprises all information about a food made available to a final consumer. So not only product labels, but also information published on company website, leaflets, advertisements etc.
Specific guidelines for mandatory information
Secondly, the Food Information Regulation provides specific guidelines for mandatory food information, such as the name of the food, the list of ingredients etc. These guidelines relate for instance to the legibility of this information, requiring a minimum font size of 1,2 mm for the x-height, defined in Annex IV to the Regulation. They also relate to the place where mandatory information is shown, requiring that such information appears directly on the package or on a label attached thereto, so not on a separate leaflet. In cases of distance selling, most of the mandatory information should be available before the purchase is concluded. i.e. it should clearly appear on the website where the food at stake is offered for sale. Furthermore, these guidelines cover language requirements, stipulating that mandatory information shall appear in a language easily understood by the consumers of the Member States where a food is marketed. For the Netherlands, this would clearly be Dutch, but the Member States also have the authority to stipulate that the particulars shall be given in one or more languages of the official languages of the European Union. Such language could be used as an alternative but also in addition to the national language.
Nutrition declarations
Thirdly, nutrition declarations have become mandatory under the Regulation. Such declarations shall imperatively include the following seven elements contained in the food product: (1) energy value, (2) amounts of fat, (3) amounts of saturates, (4) amounts of carbohydrates, (5) amount of sugar, (6) amount of proteins and (7) amounts of salt. Furthermore, they may be supplemented with another six elements such as starch and fibre. The nutrition declaration should come in the form of a table, if space permits, and may be placed on the side or on the back of a packaging (not necessarily at the front side). The information provided must be given per 100 mg or 100 ml – only in addition thereto, information per portion can be given, provided that the portion is quantified. In order to indicate reference intakes of food ingredients, quite often the term GDA (Guideline Daily Amount) is used. This is not a requirement under the Regulation, but if used, this should be done in a way consistent with the Regulation. For vitamins and minerals, it is mandatory however to use the term RDA (Recommended Daily Amount).
Conclusion
The Food Information Regulation brings about such important modifications for food information, that it is very likely that the packaging of most of the products marketed in Europe needs to be adjusted. The nutrition declarations that Google intends to publish for various foods are just one element of those modifications. It is thereby ironic that in an attempt to harmonize the rules on food information, the Food Information Regulation in the same time creates barriers to EU-wide trade, by means of the language requirements for food information. Furthermore, even if the Regulation regulates food information in great detail (e.g. font prescribed font size), it is questionable whether that framework will work for B2B transactions or in cases where the food is not sold to final consumers (e.g. mass caterers). This is certainly food for further thought. Stay tuned to FoodHealthLegal!
 On 24 September 2014 our firm organizes a seminar addressing the developments in Functional and Medical Foods. Where? Next door to our office in Pakhuis de Zwijger.
On 24 September 2014 our firm organizes a seminar addressing the developments in Functional and Medical Foods. Where? Next door to our office in Pakhuis de Zwijger.
 Food information services by Google
Food information services by Google



