COOL update
Posted: January 7, 2014 Filed under: designation of origin, Food, Information | Tags: horsemeat, labelling, meat, processed meat Leave a comment »  As announced in the post of October 30, 2013, the European Commission was expected to table a report concerning mandatory country of origin labelling (COOL) for meat used as an ingredient. In December 2013 this report was published. The report weighs the need for the consumers to be informed, the feasibility of introducing mandatory COOL and provides a cost/benefit analysis including the impact on the single market and international trade. On the basis of the debate following this report, the Commission will consider what, if any, appropriate next step should be taken. Tabling a legislative proposal concerning COOL for processed meats is a possible outcome. What was in the report?
As announced in the post of October 30, 2013, the European Commission was expected to table a report concerning mandatory country of origin labelling (COOL) for meat used as an ingredient. In December 2013 this report was published. The report weighs the need for the consumers to be informed, the feasibility of introducing mandatory COOL and provides a cost/benefit analysis including the impact on the single market and international trade. On the basis of the debate following this report, the Commission will consider what, if any, appropriate next step should be taken. Tabling a legislative proposal concerning COOL for processed meats is a possible outcome. What was in the report?
Brief description of EU supply chain of processed meats and traceability
The EU meat processing industry represents more than 13.000 companies (Food Business Operators, FBOs) being mainly SME’s (90%). Products range from relatively simple meat preparations, e.g. fresh meat with spices, to sophisticated multi-ingredient foods. SME’s tend to change their suppliers more times a year to guarantee an adequate level of raw material at an affordable price. When the FBO process meat into (multi-ingredient) foods, these are then further sold to retailers/catering/butchers. Because of the variety of suppliers the FBOs of (multi-ingredient) foods use and the relatively small quantities they order, the FBOs do not have enough bargaining power to impose origin requirements to their suppliers. The supply chain of processed meats is quite complex and lengthy. The more complex the cutting and processing stages and the more advanced level of processing, the more complex traceability becomes. The existing EU traceability systems are not adequate to pass on origin information because the legislation is primarily based on the need to ensure food safety. Information to consumers is becoming more important, but food safety is still the core of EU food law. (see Article 18 of Regulation 178/2002 and Commission Implementing Regulation 931/2011 on the traceability requirements for food of animal origin). Because of the structure of the supply chain, the absence of a significant B2B interest in origin information and the inadequacy of the current traceability systems, the implementation of transmission of origin information to the consumer will be challenging.
Consumer interest in COOL
Despite challenging implementation, no less than 90% of consumers demand COOL for processed meat, but price and quality of meat are considered more important factors affecting consumer choice. Consumers would specifically like to know the country where meat was produced. The high percentage of consumers that want COOL could partly be caused by the horsemeat scandal, given the fact that the survey for the report took place in the midst of this scandal. According to the report, the consumer wants to be informed about the origin of meat, but does not want to pay for the additional costs that would be incurred in providing that information.
Three scenarios
The report is accompanied by a Commission Staff Working Document, which underpins the report. Further, an external study commissioned by DG SANCO by the Food Chain Evaluation Consortium (FCEC) has been undertaken. Results van be found here and here. The report describes three different scenarios:
- Maintain origin labelling on a voluntary basis (status quo);
- Introduction of mandatory labelling on the basis of a) EU/non-EU indication, b) EU/specific third country indication;
- Introduction of mandatory labelling indicating the specific EU Member State or specific third country.
For scenarios 2 and 3, different modalities of processed meats have been studied for the three main categories of the products concerned in an increasing order of processing. Scenario 2 is less informative than scenario 3, as scenario 3 provides more specific origin information. Please see below for a table concerning scenarios 2 and 3.
The scenarios examined
Scenario 1 would not raise any additional operational challenges but it would not provide a fully satisfactory solution to the consumer demand for origin information. In this respect scenario 2 and 3 would both be more desirable. However, scenario 2 would result in an increase in operating costs for FBOs up to 25% and may result in market segmentation and changes in international trade flows. It would also increase the burden on public authorities with 10-30%. Under scenario 3 the increased burden on public authorities is expected to be even higher along with an increase of 8-12% of the total production costs.
Table: Advantages and disadvantages of origin modalities under scenarios 2 and 3.
| Modalities under 2nd and 3rd scenario | Advantages | Disadvantages | |
| Category I: Meat preparations/mechanically separated meat | Country where ingredient was wholly obtained or country of last substantial transformation (Customs Code) | – Provides meaningful information to the consumer;- Trimmings and fat could be used as ingredients, where origin is determined as the country of the last substantial transformation. | – Additional traceability systems;- Implementation could be challenging if multiple origins are involved;- Trimmings and fat are not likely to be used as ingredients in cases, where origin is determined as the place of minimum rearing prior to slaughter, given the challenges in storage/traceability. |
| Place of minimum rearing prior to slaughter + place of slaughter | – Places more emphasis on the provenance of the raw material where the ingredient was not wholly obtained in one country. | – Additional traceability systems;- Implementation could be challenging if multiple origins are involved;- Trimmings and fat are not likely to be used as ingredients, given the challenges in storage/traceability. | |
| Category II: Meat products | Country where ingredient was wholly obtained or country of last substantial transformation (Customs Code) | – Places more emphasis on the place of processing where the country of last substantial transformation applies;- Technically feasible for FBOs;- More practical, if multiple origins are involved;- Trimmings and fat could be used as ingredients. | – Provides no information on the provenance of the raw material where the country of last substantial transformation applies. |
| Place of minimum rearing prior to slaughter + place of slaughter | – Places more emphasis on the provenance of the raw material where the ingredient was not wholly obtained in one country. | – Provides no information on the place of processing;- Additional traceability systems;- Particularly challenging where multiple origins are involved;- Trimmings and fat are not likely to be used as ingredients, given the challenges in storage/traceability. | |
| Category III: Multi-ingredient foods with meat used as an ingredient | Country where ingredient was wholly obtained or country of last substantial transformation (Customs Code) | – Places more emphasis on the place of processing where the country of last substantial transformation applies;- Trimmings and fat could be used as ingredients. | – Provides no information on the provenance of the raw material where the country of last substantial transformation applies;- Additional traceability systems;- Particularly challenging where multiple origins are involved. |
| Place of minimum rearing prior to slaughter + place of slaughter | – Places more emphasis on the provenance of the raw material where the ingredient was not wholly obtained in one country. | – Provides no information on the place of processing;- Additional traceability systems;- Particularly challenging where multiple origins would be involved;- Trimmings and fat are not likely to be used as ingredients, given the challenges in storage/traceability. | |
Final thoughts and more upcoming changes
The overall conclusion of the report is that consumer interest in COOL is strong, but this is not reflected in the willingness pay for the extra costs for FBOs and an additional administrative burden. Further, as set out in my previous post on this subject, COOL would not prevent fraud like the horsemeat scandal from happening again at all. While being informed about the origin of the meat, the consumer still risks receiving misleading information concerning the ingredient(s) itself. However, the horsemeat scandal did show that the current traceability systems are not adequate in case of incidents. In the aftermath of the horsemeat scandal, the debate concerning COOL might therefore give rise to changes to the traceability systems. More specifically, the discussion between the Commission, the Council and the Parliament concerning COOL may result in legislative changes that have an impact on your business. Aside the report discussed in this post, the Commission is also expected to adopt implementing rules on mandatory COOL for unprocessed meat of sheep, goat, pig and poultry, based on the New Labelling Regulation. Get informed and subscribe to updates from FoodHealthLegal (see section above ‘Tweets” on the right side of your screen) in order to stay posted!
Dutch interim injunction with high impact
Posted: November 27, 2013 Filed under: Food, insurance, reimbursement | Tags: healthcare insurance, medical, reimbursement Leave a comment »On November 15, 2013, the District Court Gelderland rendered its judgment in the lawsuit (interim injunction) that was filed by Nutricia against healthcare insurer VGZ and five other insurers. The court ruled that the reimbursement policy VGZ wants to implement in 2014 regarding medical nutrition is in violation of the Dutch Health Insurance Act. If you are a company that produces medical nutrition or other products that are subject to reimbursement by a healthcare insurer, continue reading!
Reimbursement policy medical nutritional drinks
The healthcare market recognizes a clear need for medical nutrition. For instance, patients that are so weakened that they are unable to eat normally, or people with food intolerances use medical nutritional drinks. Patients are entitled to reimbursement of medical nutritional drinks under the conditions set out in the Health Insurance Act. In order to meet the obligations under the healthcare insurance policy, VGZ closed so-called “care agreements” with suppliers that deliver the drinks to the patients. The care agreements include provisions concerning the compensation that is paid for the delivery of medical nutritional drinks to insured patients. Suppliers, in turn, conclude contracts with manufacturers that offer the medical nutritional drinks. Nutricia is one of the manufacturers of medical nutritional drinks intended for patients to whom the use of special nutritional drinks is prescribed by a doctor or dietician.
The insurer’s preferential policy
Healthcare costs are a growing concern in western societies. From this perspective, cutting down oversized margins that unnecessarily increase the price of health care products is a key objective for each insurer. To that end, VGZ adopted a new reimbursement policy for 2014. Under this new preferential policy, medical nutritional drinks were divided into four clusters. For each cluster of interchangeable products a preferred product (that will be fully reimbursed by VGZ) was appointed through a tender to which manufacturers could subscribe. The German manufacturer Fresenius Kabi won the tender. Other products in the same cluster that are equivalent to the preferred product are only reimbursed if there is a clear medical indication. Equivalence is established based on the same nutritional composition, active ingredient and dosage.
Maximum reimbursements set by the insurer
For other nutritional drinks that are not interchangeable, including Nutricia’s Nutridrink, a maximum reimbursement applies. The maximum reimbursement means a high discount (48 to 56 %) on the purchase price that the manufacturer can charge to the supplier according to the price per drink that is yearly published in the G-Standaard. For Nutricia this practice means that they are ‘being pushed out of the market’. As it is unlikely that the suppliers will be prepared to pay a price higher than reimbursement level, Nutricia fears it will need to sell its products below cost-price.
Claims made by Nutriticia’s and by patient and dietician organizations
Nutricia’s claims in these proceedings were in particular aimed at the arbitrary way in which the maximum reimbursement prices regarding non-interchangeable products were set. Two organizations representing patients and nutritionists that joined Nutricia in these proceedings opposed the preferential system that VGZ had set regarding the products that were considered interchangeable. In particular, they opposed VGZ’s requirement that in at least 75 % of the cases, the pharmacist should deliver the preferred product to the patient. Appointing preferred products by healthcare insurers is an allowed common practice in case of medicinal products. But does this mean this practice is also allowed for nutritional drinks?
The outcome: reimbursement policy held unlawful
The answer to the questions above: “No”. The required delivery of 75 % preferred products didn’t go down well at the court. According to the court, the preferential policy does in fact limit the claim that patients have to reimbursement of medical nutritional drinks. This is unlawful vis-à-vis the pateints at stake. The clustering of nutritional drinks into four groups and the appointment of preferred products is contrary to the Health Insurance Act (and the regulations based on this Act). Other than in respect of medicinal products, the Health Insurance Act does not provide for the possibility to adopt a preferred product policy regarding reimbursement of medical nutritional drinks. This is also evident from this document from the Dutch Healthcare Authority (Nza). The rationale behind is that interchangeability that can be established for medicinal products based on active ingredients cannot just simply be applied to medical nutrition, as various other factors need to be taken into account. Furthermore VGZ is not allowed to adopt any arbitrary discounts to curb costs regarding products considered non-interchangeable, in as far as these discounts do not take into account the cost-price of these product but are mainly aimed at discouraging the supply thereof. This is completely arbitrary and unlawful vis-à-vis the manufacturer. The court ruled that VGZ is not allowed to further execute or conclude care agreements for medical dietary nutritional drinks implementing a reimbursement policy along these lines.
No final decision
Appeal against this 15 November 2013 interim injunction is open until 8 February 2014. However, even if this is not a final decision, it has proved being effective for Nutricia, as the disputed reimbursement policy cannot be implemented for 2014. Although the purpose of limiting healthcare costs is a common concern, this decision has shown that the end does not always justify the means.
Prošek vs. Prosecco – cheers!
Posted: November 13, 2013 Filed under: designation of origin, Food, geographical indication, Information | Tags: designation of origin, DO, geographical indication, GI, prosecco, Prošek, protection Leave a comment »Italy vs. Croatia
What’s in a name? With Croatia’s accession to the European Union as of 1 July 2013 the beloved Croatian wine called Prošek is not allowed to be marketed under the name ‘Prošek’ anymore. How come? The Italian wines marketed under the name ‘Prosecco’ enjoy the rights granted by the protection of Designation of Origin since 17 July 2009. Meanwhile, the Croatian Prošek wine, even though it has been produced since hundreds of years ago, enjoys no such protection or protection as a “geographical indication” protection, due to the failure of the Croatian national authorities to protect this name.
Legal background
EC Regulation No. 1234/2007 (Single CMO Regulation) lays down the rules on the protection of “designation of origin” (DO) and “geographical indication” (GI) of wines marketed within the EU, independent whether they originate from an EU Member State or a third country. The DO or GI protection of wines originating from third countries (as Croatia was before joining the EU) is possible without going through the entire procedure set out in the Single CMO Regulation, provided that such protection is initially granted on national level (Article 118d). Croatian Prošek was not protected on national level and therefore could not obtain protection on EU level anymore.
No focus on protection of the names of local products
In order to convince the EU for its membership in the Union, Croatia had to solve several issues. Clearly, during that period there was no focus on protection of products, such as Prošek, on EU level. In fact, besides the Prošek wine, many other Croatian products (http://www.croatiaweek.com/no-croatian-products-with-european-protection/) lack protection on EU level. This might be the result of a weak IP protection policy, which maybe characterizes not only Croatia but also other western Balkan countries that aspire EU membership such as Albania, Bosnia and Herzegovina, Kosovo, Montenegro, Serbia and The Former Yugoslav Republic of Macedonia. This case should serve as a lesson to those countries so that they ensure that their products are properly protected before their eventual entry into the EU. See also this article.
Back to the bottles
Coming back to the Processo and Prošek dispute, why is it forbidden to use the name Prošek for Croatian sweet wine since 1 July 2013? Italian Prosecco producers claimed that the name Prošek is too similar to their already protected name. The name Prošek might therefore confuse those consumers who intend to buy Prosecco but end up buying Prošek. Such claims were followed by the threats of the Italian Prosecco wine producers about complaining to the EU authorities in case the Croatian wine producers would continue to market their wines under the name Prošek.
Confusion indeed?
One could argue that the first paragraph of article 118j of the Single CMO Regulation allows the registration of a name that is wholly or partially homonymous with that of a name already registered. However, this argument will not be successful as the local and traditional usage and the risk of confusion are decisive factors in determining a permissible registration. The traditional usage of the name Prošek and this wine`s qualities and differences when compared to Prosecco may be found here, here and here. Looking at the differences between the sweet Croatian Prošek and the sparkling Italian Prosecco, I couldn’t help but wonder how a consumer would confuse a bottle of Prošek with a bottle of Prosecco.
Conclusion: take up that challenge!
The Croatian authorities gave the impression that they gave up trying to protect the name Prošek, without fighting for it. The threat of being sued for marketing the Croatian wine under the name Prošek does not necessarily mean that the European court would decide in disfavor of Croatia. The Court of Justice has to decide whether or not the names Prosecco and Prošek may co-exist (as in the similar case with the Hungarian “Tokaj” and the Italian “Tocai” (See joined cases C-23/07 and C-24/07, ‘Confcooperative Friuli Venezia Giulia and Others’).
The author is grateful to Arber Gjunkshi, paralegal at Axon Lawyers, for his valuable contribution to this post.
New Labelling Regulation: more COOL?
Posted: October 30, 2013 Filed under: Food, Information | Tags: country-of-origin, horsemeat, information, labelling Leave a comment »Rumour has it that the European Commission will decide not to go for full country-of-origin labelling (‘COOL’) on all meat products in the European Union. According to globalmeatnews.com the European Commission is planning to recommend only partial country-of-origin labelling for fresh meats. This is not in line with the request of MEPs and EU ministers who have explicitly asked to beef up the new Labelling Regulation with better meat origin labelling for both unprocessed and processed meats. Why does the Parliament pressure the Commission to impose origin-labels on processed meat?
The reason for the Parliament’s pressure: the horsemeat scandal
The horsemeat scandal has been making headline news over the last year. It was a scandal caused by fraudulent labelling. Horsemeat was present in beef products and the consumer was not informed about this. For a Dutch perspective on the horsemeat scandal, see this article (in Dutch).
To restore consumer confidence and to improve controls the Commission has launched an Action Plan. COOL is part of the Action Plan and the Parliament has been pushing the Commission to adopt mandatory origin labelling for both fresh meat and meat in processed food (meat as an ingredient). In April, and later in September 2013, MEP Glenis Willmott urged the Commission to put rules on country-of-origin in place on ‘country of origin’ labelling for meat in ready meals and in processed foods. This month Health Commissioner Tonio Borg received a letter from MEP Agnès Le Brun to further pressure the European Commission for better meat origin labelling. The MEPs main arguments are that the horsemeat scandal highlighted the need for honest food labelling and by using COOL producers would have a much better grip on their supply chain.
Could COOL ensure honest labelling and prevent fraud?
In March 2013 Commissioner Tonio Borg answered this question in an interview. According to Borg the horsemeat scandal should be seen for what it is: a fraud rather than a demonstration of a regulatory gap. COOL would not necessarily create another hurdle for fraudsters: “(…) one could be honest about the origin but fraudulent about the labelling on the ingredients.” If you want to see the video of the interview, click here. I agree with Borg. The horsemeat scandal was a case of intended mislabelling, COOL will not prevent it from happening again.
Country-of-origin: current EU legislation
The current Labelling Directive only requires the place of origin or provenance to be mentioned on the label where failure to give this information might mislead the consumer (article 3(8)). In the EU, the origin must always be labelled for olive oil, fish (unless it is canned or prepared), beef, fresh or frozen poultry of non-EU origin, wine, most fresh fruit and vegetables, honey and eggs. For all other foods, origin labelling is optional.
New Labelling Regulation
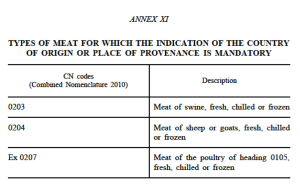
According to article 26 of the new Labelling Regulation, indication of the country of origin shall be mandatory where failure to indicate this might mislead the consumer and for meat listed in Annex XI (see below).
The following steps have to be taken by the European Commission from now:
Autumn 2013 – Adopt a Commission report on the possibility to extend mandatory origin labelling of all types of meat used as ingredient in foods and take any necessary follow up action.
December 2013 – Adopt implementing rules on the mandatory origin labelling of unprocessed meat of sheep, goat, pig and poultry, based on the new Labelling Regulation.
December 2013 – Adopt implementing rules to prevent misleading use of voluntary origin labelling in foods, based on the Regulation on Food information to consumers.
December 2014 – Adopt Commission reports, based on the new Labelling Regulation, on the possibility to extend mandatory origin labelling to:
- other unprocessed meats not already covered by mandatory origin labelling rules, such as horse, rabbit, game meat etc.;
- milk;
- milk as an ingredient in dairy products;
- single ingredient foods;
- unprocessed foods;
- ingredients that represent more than 50% of a food.
So far, no official report from the European Commission on the meat labelling subject has been released, but it is expected to be published soon. Anyway, you will see an update here as soon as the report is published.
More COOL?
The Commission has not given a clear position on COOL yet, but if the rumours are true, the Commission is set to propose COOL for fresh pork, poultry and lamb. Compared to the current legislation, this results in more COOL because the requirements are being rolled out from beef to other meats, which will have an impact on the meat industry. But not totally COOL as the Parliament wants to see it. At this point it looks like COOL for processed meat will be rejected, which probably will be substantiated through an impact assessment. COOL for processed meat is likely to turn out to be too costly for the industry, and the consumer might not want to pay the price for origin information. Of course this is still speculative. Stay tuned for the update!
How novel is your food?
Posted: September 6, 2013 Filed under: Food, novel food | Tags: food, guidance, novel, novel food Leave a comment » If you like to add a new ingredient to – for example – a beverage or want to start selling ‘superberries’ or an exotic seed from Asia in the EU, you should always check whether the food is a so-called novel food. The producer, importer or any other person responsible for the placing of a product on the EU market is primarily responsible for compliance with the EU-legislation, including the Novel Foods Regulation.
If you like to add a new ingredient to – for example – a beverage or want to start selling ‘superberries’ or an exotic seed from Asia in the EU, you should always check whether the food is a so-called novel food. The producer, importer or any other person responsible for the placing of a product on the EU market is primarily responsible for compliance with the EU-legislation, including the Novel Foods Regulation.
According to this Regulation, novel foods require pre-market approval in the EU. But when is a food product actually a novel food? Based on a steady stream of questions, our firm has noticed clients struggle with this question. Good news: this guidance document, recently published by the European Commission brings some relief.
Novel Foods Regulation
Food products (including ingredients) that have not been used to a significant degree for human consumption within the EU prior to the entry into force of the Novel Foods Regulation on 15 May 1997, are, in general, considered novel foods and they should fall in one of the following categories:
- foods and food ingredients with a new or intentionally modified primary molecular structure;
- foods and food ingredients consisting of or isolated from micro-organisms, fungi or algae;
- foods and food ingredients consisting of or isolated from plants and food ingredients isolated from animals (except for foods and food ingredients obtained by traditional propagating or breeding practices and having a history of safe food use);
- foods and food ingredients to which has been applied a production process not currently used, where that process gives rise to significant changes in the composition or structure of the foods or food ingredients which affect their nutritional value, metabolism or level of undesirable substances.
The Regulation does not apply to food additives, flavourings and extraction solvents used in the production of foodstuffs. Furthermore genetically modified organisms fall outside the scope of the Regulation since the Regulation on genetically modified food and feed that entered into force on 18 april 2004.
Human consumption to a significant degree
A food product is not a novel food within the meaning of the Regulation if it has been used for human consumption to a significant degree in the EU. What is a ‘significant degree’?
This is where the guidance document from the European Commission is helpful. It includes a decision tree and questionnaire to help you assess your product.
The facts to support that a food has already been used for human consumption to a significant degree within the European Union before 15 May 1997 should be based on robust, reliable information. Data must be taken from referenced sources and must relate to foods which have been legally on the Community market.
All available data and information should be taken into account in establishing whether the food product in question falls within the scope the Regulation. These could include for example, recipes, cookbooks, catalogues and sales data. Also (national) legislation regulating the food in question should be taken into account.
Food products commonly used and known in different EU Member States and in specific regions vary greatly. According to the EC guidance, an established history of food use to a significant degree in at least one EU Member State is sufficient to exclude the food from the scope of the Regulation. Obviously, the more a food has been used the easier it is to demonstrate ‘a significant degree’ of use.
Borderline products: Food/Drugs/Cosmetics
Products that have been used for their medicinal effects or as cosmetics are not considered novel foods. The food has to be used for the purpose of food, and not otherwise. Also, the classification of products as medicinal product or a food can differ across the EU because a food product can be classified as a medicinal product in one Member State and as food in another.
Use in food supplements
The Standing Committee on the Food Chain and Animal Health agreed in its meeting of 14 February 2005, that a use exclusively in food supplements before 15 May 1997 would not be considered as “human consumption to a significant degree”. Authorisation under the Regulation would be necessary if the food product has been exclusively used in food supplements.
Herbals considered as food
Significant use of a food product within Europe can be substantiated if the food product is listed on the ‘Inventory list of herbals considered as food’. This list has been drawn up by the European Herbal Infusions Association (EHIA), and is based on the habits, the traditions and/or the regulatory status of plants in different member states. EHIA’s list can be regarded as a compendium of the different histories of use all over Europe. This makes this list a useful resource because it helps to identify whether certain plant material has been used as food ingredient in Europe.
Novel Food Catalogue
In addition, the Novel Food Catalogue of the European Commission can be consulted. The Novel Food Catalogue is a non-exhaustive list of products of plant and animal origin and other substances subject to the Regulation. The list is a living document that is amended with novel foods earmarked by EU member states and the Commission in the Novel Food Working Group. The catalogue provides important guidance as to whether a product will require authorization under the Regulation. If a food product is not listed, this supports the conclusion that the product is not covered by the Regulation.
Useful documentation on Member State-level
Some EU countries have extensively documented which plants/herbs are considered safe and are allowed to be marketed as food. This documentation also gives insight into whether a food product is allowed and whether it is regarded as a novel food that is subject to the Regulation.
For example Belgium and Germany use detailed lists of allowed plants. The German list is helpful to determine whether a plant/herb is considered as food, drug or novel food.
Belgium has recently updated their indicative lists of plants, plant parts and substances and their novel food status. On the Belgian website you can also find a link to a convenient PDF-file named ‘Tools to prove that a food or food ingredient is not a novel food’.
In case of doubt, you may consider consulting the relevant competent authority for novel foods on the status of your food product. But you don’t want to spill the beans; always think it through before contacting authorities because they cannot un-know what you tell them about your product. An example of a review on the status of goji berries by UK’s Food Standards Agency can be found here.
Tools for the novel food toolbox
Are you familiar with similar lists such as the lists used in Belgium and Germany or other documents (preferable in English) that are helpful in the process of determining the status of a food product in the light of the Regulation or other convenient tools? Great! Your comment would be appreciated.
Best check before
Why do you have to check if the food product you are planning to sell on the EU market is a novel food? As mentioned in the beginning of this post, novel foods require pre-market approval. A non-compliant food product is illegal and liability-issues and/or fines might end up on your plate. Better check before you market, and use the tools mentioned in this post to help you find the answer.
Smarter rules for safer food: draft regulation on official controls
Posted: July 8, 2013 Filed under: Enforcement, Food | Tags: animal health, food, food safety, official controls, plant health Leave a comment » On the 6th May 2013, the European Commission adopted a proposal for a draft regulation on official controls and other official activities performed which aims to strengthen the enforcement of health and safety standards across the “agri-food chain”.
On the 6th May 2013, the European Commission adopted a proposal for a draft regulation on official controls and other official activities performed which aims to strengthen the enforcement of health and safety standards across the “agri-food chain”.
This is an important Regulation, which will have an impact on every food business, producer, operator and Competent Authority in the EU.
Currently, the legislative framework for the organization of official controls is established through Regulation (EC) No. 882/2004. The new proposal aims to put in place a more robust, transparent and sustainable regulatory framework that is better “fit for purpose” which will repeal Regulation (EC) No 882/2004.
The proposal is part of a ‘landmark package’ which also includes major reviews to modernise, simplify and strengthen animal health, plant health and plant reproductive material legislation. The current body of EU legislation covering the food chain consists of almost 70 pieces of legislation. The package of reform will cut this down to 5 pieces of legislation. For official controls this is achieved by integrating the current applicable rules in specific areas currently governed by separate sets of rules (e.g. controls on residues of veterinary medicinal products in live animals and animal products, and plant health controls) into the framework of the new official controls Regulation.
Watch this video for the statement of Tonio Borg, Member of the EC in charge of Health and Consumer Policy, concerning this major reform.
An overview of legislation that will be repealed or amended through the proposed Regulation can be found through this link (see page 2). More information can be found here, here and here.
Some highlights
- Broadened scope to include the whole agri-food chain. The new areas included are plant health, plant reproductive material and animal by products;
- The current system of mandatory fees to finance the effective implementation of these controls within a sustainable system along the whole chain will be extended to other sectors within the chain which are currently not charged; Micro-enterprises will be exempted from such fees, but not from controls, in order not to affect their competitiveness;
- Member States will also be asked to fully integrate anti-fraud checks into their national control plans and to ensure that financial penalties applicable to violations must at least offset the economic advantage sought through the violation;
- Provisions to enhance transparency in relation to how competent authorities carry out official controls. This includes a requirement to publish more timely and regular information on the types of controls and their outcome and to establish “rating schemes” whereby consumers can consult data on the performance of retailers restaurants and other businesses;
- The creation of a common framework for carrying out border import controls on animals and goods entering the EU;
- Provisions allowing the Commission to adopt detailed rules across a range of areas of official controls via delegated and Implementing Acts;
- Rules to facilitate official controls for on-line sales. These facilitate competent authorities to order samples on line for official testing without having to identify themselves. The Regulation also specifically gives competent authorities the option of closing internet sites where non compliances have been identified relating to activities of the site.
Entry into force
Approximately in 2016. It depends on when both European Parliament and Council have approved the final text of a legislative proposal. More information about the legislative procedures? Click here for general information and here for a PDF-file concerning the ‘Ordinary legislative procedure’.
Next step?
This letter was sent to the European Parliament and the Council. They will consider the Commission’s package of measures and adopt their positions. When more details of the legislative planning or other news regarding this proposal are published, you will read it here!