Posted: October 7, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Authors, clean meat, cultivated meat, Food, Information |
 On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
Legal names can be set, but the French Decree does not contain legal names
The ECJ starts the motivation of its decision with acknowledging that the FIC Regulation leaves room to adopt legal names at Member State level where such do not exist at EU level. Where legal names are set, these cannot be used for products not complying with the specifications of that name. As an example, the ECJ refers to the term ‘meat’, which is legally defined as ‘the edible parts of animals’. A food not containing such parts can therefore not use the name ‘meat’, even if it is accompanied by specifying terms such as ‘vegetarian’. The same applies to milk and certain milk products, for which the legal name is laid down in the COM Regulation. Indeed, as we know well from the Tofutown decision, names such as ‘plant-based milk’ are a no-go.
According to AG Capeta, the French Decree under attack established legal names. This was done on the one hand by establishing a list of meaty names of which the use is prohibited for the designation of their plant-based counterpart (such as steak), and on the other hand by authorizing the use of certain meaty names for foods containing vegetable proteins provided that they do not exceed a certain proportion (such as cordon bleu (maximum 3,5 % vegetable protein)).
The ECJ ruled however differently. In the first place, it recalls that the French authorities themselves rejected the hypothesis that Decree No 2022-947 lays down a legal name. Therefore, the learnings from the Tofutown decision cannot be applied to the case at hand. In the second place, it also states that legal names must, according to the definition thereof in the FIC Regulation, be defined in order to designate a foodstuff. The adoption of a legal name thus means associating a specific expression with a given food. This is done by setting certain conditions, especially with regard to the composition of the food. The French Decree contains a measure prohibiting the use of certain meaty names, which are not legally defined by the Decree, for plant-based foods. This is not the same.
Use of customary and descriptive names fully harmonized
Given that there are no legal names for plant-based foods at EU level and neither in France as far as is known to the ECJ based on the file of the case, plant-based foods must be indicated by their customary name or descriptive name. Where a customary name is the accepted name of the food that does not need further explanation, a descriptive name must explain the main characteristics of the food.
 Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
No distinction between domestic and imported products
Where the AG made in her opinion a distinction between rules covering domestic production and rules covering production abroad, this topic was no longer covered in the ECJ’s preliminary ruling. The question whether the French Decree could only apply to foods manufactured in its territory was initiated by the highest French administrative court (“Conseil d’Etat”), but became redundant since the ECJ came to the conclusion that national measures regulating or prohibiting the use of meaty names for plant-based products (other than by means of legal names) is not allowed in the first place.
We nevertheless conclude from the case that since the limitative legislation applying at a national level is not considered in conformity with Union legislation, this surely goes for national legislation applying similar restrictions on a Union level. This is justified by the fact that the ECJ recalls at the beginning of its decision the two paramount principles of Union legislation. In addition to consumer protection, this is also the smooth functioning of the internal market.
Outlook for the alternative protein sector a whole
Now the ECJ has given its interpretation of the FIC Regulation in response to the questions of the French referring court, it is now up to the latter to decide the dispute at national level in accordance with the ECJ’s preliminary ruling.
It should be noted that the ECJ’s ruling is similarly binding on other national courts or tribunals before which a similar issue is raised. This means that the prohibition to enact national measures regulating or prohibiting the use of meaty names in the absence of legal names in principle also applies to other meat analogues such as cultivated meat and those produced by precision fermentation. Having said that, these products may face other challenges, such as the question whether such products can actually be called meat (for which they must be edible parts of animals as indicated above), and to what extent the production technique used must be indicated in accordance with art. 7(1)(a) of, and Annex VI, Part A, point 1 to, the FIC Regulation. We will keep you posted!
Posted: September 20, 2024 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food |
Under the new packaging law, sustainability is no longer a ‘nice to have’ but a ‘need to have’. While companies currently distinguish themselves with recyclable and reusable packaging, this becomes the new normal under the Packaging and Packaging Waste Regulation (‘PPWR’). The PPWR is not yet formally adopted, though informal agreement has been reached at European level on the legislative proposal that was published by the European Commission in November 2022. The new regulation is expected to enter into force later this year.
The PPWR contributes to the transition towards a circular economy and builds on the current Packaging and Packaging Waste Directive, which it will replace. The first major difference can be seen in the mechanism of the law. It is no longer a directive that imposes obligations on Member States, but directly addresses economic operators such as food businesses. This includes shops and catering establishments offering refill and reuse items. The PPWR is a comprehensive piece of legislation. In this blogpost, we list three sustainability requirements under the PPWR that food businesses will face.
(1) Recyclability
One of the first sustainability requirements mentioned in the PPWR is recyclability. This goal must be achieved in two steps. From 2030 onwards, packaging must be designed to be recyclable. A list of criteria showing when this requirement is met is yet to follow. This may mean, for example, that certain hazardous substances that affect the recycling process and make the recycled material unsafe are no longer allowed in packaging. Five years later, from 2035, packaging should actually be recycled in practice. The abovementioned entry deadlines will shift in case the European Commission does not publish on time its clarifying rules as required under the regulation.
For now, food packaging intended for vulnerable groups (think about persons using medical food and baby food), among others, is exempted. For innovative packaging that cannot meet the recyclability requirements taking into account current collection and recycling methods, a temporary five-year exemption can be applied for at national level.
(2) Percentage of recycled material
Plastic packaging still contains relatively little recycled material. In view of the European circularity targets, minimum percentages are now set for this. Taking into account the limited recycling possibilities and food safety, a lower minimum percentage will apply to food packaging than to non-food packaging (namely 30% and 10% from 2030 for PET and non-PET food packaging respectively, compared to 35% from 2030 for non-food packaging). With regard to single-use plastic beverage bottles, the PPWR will replace the minimum percentage of recycled material set for such in the Single Use Plastics Directive as from 2030 (30% by that year). From 2040, the minimum percentages will be further increased for all categories.
How the percentage of recycled plastic in packaging shall be calculated exactly is still to be defined. In any case, exceptions also apply to this sustainability requirement, especially where food safety may be at stake. Packaging with a plastic part representing less than 5% of the total weight of the whole packaging unit is also exempted from the mandatory minimum percentage.
 (3) Compostable
(3) Compostable
Three years after the PPWR comes into force, certain packaging may only be offered in compostable form. In contrast, other packaging may explicitly not be marketed as such, unless such packaging was subject to national compostability requirements before the date of application of the PPWR. The compostability requirement will initially apply to sticky labels attached to fruit and vegetables, and to packaging like permeable tea bags and coffee pads. Aluminum coffee capsules are thus not covered. Non-permeable coffee capsules made of other material may be accepted for composting at national level.
‘Compostable’ under the PPWR means that the packaging complies with the European standard for industrial composting (13432). However, this standard does not match real life industrial composting and will therefore be updated. Member States may as well require that the packaging complies with the standard for home composting. Needless to say, this is a tougher standard. For the time being, the Netherlands has not indicated its intention to make use of this policy freedom.
Chain responsibility
The responsibility to meet the above sustainability requirements lies primarily with manufacturers. These are the (legal) persons who manufacture packaging or a packaged product, or who have such designed or manufactured under their own name or trademark (save for some exceptions). A food company that manufactures (itself) or has manufactured (by a third party) packaged food products is therefore a manufacturer under the PPWR. Manufacturers will have to carry out a conformity assessment. In doing so, they guarantee and declare under their own responsibility that the packaging complies with the relevant provisions of the PPWR. As part of this, the manufacturer shall draw up a dossier demonstrating compliance.
Food companies importing packaging or packaged products from third countries (importers) will have the responsibility to check whether such packaging complies with the Union rules laid down in the PPWR. Should there be a suspicion that the packaging does not comply with the law, this must be corrected. Importers must in such case also inform the competent authority. To avoid misunderstandings and conflicting interests, it is recommended that food companies make contractual agreements on this with their suppliers and/or customers. This also applies to food companies that further market packaging or packaged products on the Union market (distributors). Distributors should also actively ensure that the packaging meets the labelling requirements under the PWWR. This includes facilitating the correct disposal route and combating ‘greenwashing’.
 PPWR: Dynamic legislation
PPWR: Dynamic legislation
The PPWR is a dynamic legislative document. Several criteria still need to be fleshed out in secondary legislation and the PPWR leaves a lot of room for evaluation, additions and deletions (the latter especially with regard to the exemptions it currently contains). However, sitting still and waiting for more clarity is not recommended since developments are moving fast. Our advice: take stock of the current situation within your company, start talking to your suppliers and customers, and check whether current contracts need to be adjusted. And above all, evaluate which sustainability improvements can be made. Because one thing is certain: the EU needs to step up its efforts to become climate neutral by 2050.
A Dutch version of this blogpost is available at VMT.nl.
All images are by freepik.
Posted: July 23, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Food, Information |
 Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
What is the case about? Advertiser, a food business marketing infant and follow-on formula, published statements on its website about its products such as:
- “Unique combination of natural lactic acid cultures and valuable fibers.”
- “Where organic ends, […] continues: […] guarantees an excellent quality and supersedes the legal requirements concerning organic.”
- “Inspired by nature.”
- “With the first milk that is non mom’s, I want to do all well” (next to a picture of a baby in the arms of a woman).
The question is whether (a) these texts qualify as advertisements, and (b) whether the texts give the impression that the follow-on formula is as good as breastmilk.
What is the legal rule? Consumer-oriented advertising for infant formula is prohibited. Advertisements for follow-on formula are possible, but should not dissuade or discourage breastfeeding. This is laid down in articles 4.1 and 5.2 of the Dutch Advertising Code for Infant Formula, in line with EU Regulation 2016/127. As follows from article 6(6) of aforementioned Regulation, the labelling, presentation and advertising of infant and follow-on formula shall not include terms like ‘humanized’, ‘maternalized’ or ‘adapted’. The Dutch Advertising Code for Infant Formula further lists ‘inspired by breastmilk’, ‘protected effect of breastmilk’, and ‘contains nutrients that are also found in breastmilk’ as examples of prohibited statements to avoid discouragement of breastfeeding.
What was decided? The Board of Appeal upheld the decision of the Advertising Code Committee and ruled that (a) the texts on advertiser’s website are clearly promotional in nature, and that (b) the consumer will understand the references to nature to be references to breastmilk (and not to the products having organic qualities, as was explained by advertiser). As this is in violation of the Dutch Advertising Code for Infant Formula, advertiser is requested to no longer make such advertisements.
 Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Looking into the future, it is interesting to note that the same rules shall in principle apply to cultivated breastmilk made in a lab. Various companies are already working on this concept such as French Nūmi and BIOMILQ in the US. Giants like Danone and FrieslandCampina have announced strategic partnerships with cell-based human milk component start-ups. Companies working on such substitute products obviously want to (and should) explain their products to the public. Statements such as ‘inspired by breastmilk’ and ‘contains nutrients that are also found in breastmilk’ may not seem unreasonable in this context. Currently this is however not allowed. Will the Dutch self-regulatory code (which is more specific in prohibited terms than EU Regulation 2016/127 and the WHO Code) be updated, so that such innovations can be appropriately explained to the public? Stay tuned – we will keep you posted!
The full case can be read here (in Dutch).
Posted: April 24, 2024 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food, Food Supplements, Health claims, Information, Nutrition claims |
 The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
When entering the keyword ‘probiotic’ in the EU Health Claims Register, one is confronted with over 100 rejected health claims. Over the past years, this has led to the question whether the term ‘probiotic’ should be allowed under certain conditions, and has resulted into divergent policies in different EU member states. To protect both the food industry (against unfair competition) and the consumer (against misleading information), these divergent policies are reason for IPA Europe to call for a harmonized framework.
Czech Republic, Northern Ireland and France
Although EU member states generally consider ‘probiotics’ to be an unauthorized health claim under the EU Claims Regulation, some EU member states take a different approach. ‘Probiotics’ is for example considered a nutrition claim in the Czech Republic. Such claim is allowed if the conditions set forth in the EU Claims Regulation are met. This means, amongst others, that the good bacteria in question are present in the food in an amount that, according to generally accepted scientific evidence, causes the claimed beneficial effect. By contrast, in France and Northern Ireland, the term ‘probiotics’ can be used as a general, non-specific health claim that is allowed in combination with the authorized health claim “live cultures in yoghurt or fermented milk improve lactose digestion of the product in individuals who have difficulty digesting lactose”.
Italy
Italy takes the view that the term ‘probiotics’ does not meet the definition of a health claim and therefore falls outside the scope of the EU Claims Regulation. Italy supports this view by EFSA’s conclusion that probiotic colonization in the intestinal flora (without further specification of bacterial species or strains) is insufficient evidence to substantiate a beneficial effect on human health. In other words, no link between probiotics and health can be demonstrated, for which reason ‘probiotics’ cannot be considered a health claim. Aforementioned reasoning is however not a free pass to unconditionally use the term ‘probiotics’ in Italy. Instead, the following conditions must be met if and when using this term: (i) safety for human consumption, (ii) a history of use for the benefit of the intestinal flora, and (iii) presence of the relevant bacteria in the food in live form and in an adequate quantity until end of shelf life.
Denmark and Spain
In Denmark, the term ‘probiotics’ can be used based on a different legal ground, namely as a mandatory category designation under the EU Food Supplements Directive. In France, such is also possible. Indeed, Article 6(3)(a) of the EU Food Supplements Directive requires that the labeling of the food supplement shall bear “the names of the categories of nutrients or substances that characterize the product or an indication of the nature of those nutrients or substances”. In Spain, the claim ‘probiotics’ is allowed thanks to the principle of mutual recognition. Based on this principle, a product lawfully marketed in one EU member state must also be accepted on the market of another EU member state. Since probiotic claims are therefore allowed on the Spanish market for products from other EU member states, banning the term ‘probiotics’ at national level would discriminate against national producers. Therefore, both food products produced within Spain and those produced outside of the country can bear the claim ‘probiotics’.
Netherlands
In the Netherlands – just like in Denmark and France – the term ‘probiotics’ can be used as a category designation for food supplements. This Dutch practice is laid down in the Guideline Document on the EU Claims Regulation by the Dutch Health Advertising Knowledge and Advice Council (in Dutch: Keuringsraad). An earlier version of the Nutrition and Health Claims Manual by the Dutch Food Safety Authority (in Dutch: NVWA Handboek Voedings- en Gezondheidsclaims) also explicitly mentioned this possibility. Such is now longer mentioned in the latest version of the Nutrition and Health Claims Manual, which can be explained by the fact that ‘probiotics’ as a category designation for food supplements is not a nutrition or health claim.
Although claims that further elaborate on the health effect of probiotics (sporadically) occur on the Dutch (online) market, these are not allowed. Whether the expression “increases the good bacteria in the intestinal flora” (without using the term ‘probiotics’) is acceptable in the Netherlands, is yet unclear. Yakult uses this expression to advertise its fermented milk drink. The Dutch Health Advertising Knowledge and Advice Council would not allow this expression in the context of its preventive supervision in the context of food supplements, because this expression in effect creates a link between the food product and health. If the NVWA has however a different opinion on this and does consider aforementioned expression possible, then other food businesses can take advantage of this too. The call for harmonized uses of the term ‘probiotics’ by IPA Europe, about which more below, can possibly contribute to the acceptance of such expression at national level.
”Probiotics’ not a health claim”
As demonstrated above, various EU member states have introduced national rules on the use of the term ‘probiotics’. As a result, IPA Europe believes that the European Commission’s position that ‘probiotics’ implies a health benefit and is therefore a(n unauthorized) health claim no longer holds water. Instead, IPA Europe advocates qualifying the claim ‘contains probiotics’ as a nutrition claim, just like ‘contains vitamins and minerals’ and ‘contains fiber’. Aforementioned substances may have beneficial nutritional properties, but no specific health benefit is claimed. To strengthen its argument that ‘probiotics’ is not a health claim, IPA Europe explains that the term is not sufficiently precise to substantiate the claimed health benefit under reference to an EFSA guidance document published in 2016.
Call for harmonized use of the term ‘probiotics’
According to IPA Europe, ‘probiotics’ is therefore not a health claim and does not require authorization under the EU Claims Regulation. At the same time, it emphasizes the need for clear rules on the use of the claim as this contributes to a fair competitive environment for food businesses and helps consumers to make informed choices. In December 2023, IPA Europe therefore called for a clearly defined framework for the use of the term ‘probiotics’ in the EU. IPA Europe recommends the following four criteria for consistent use thereof:
- characterization of the species level and identification of at strain level;
- the probiotic strain must be safe for the intended use, e.g. based on the QPS list;
- the probiotic status should be scientifically documented; and
- the probiotic strains must be alive in the product and in a sufficient amount up to the end of shelf-life.
Final comment
The EU knows a fragmented regulatory landscape when it comes to the use of the term ‘probiotics’. Despite IPA Europe’s efforts for a harmonized approach throughout the EU, we will need European legislation or a ruling from the European Court of Justice to have the same rules in all EU member states. For now, the term ‘probiotics’ is (fortunately) not completely banned in the Netherlands and neither in quite a few other EU Member States.
This blogpost has also been published in Dutch at VMT.nl.
Posted: December 8, 2023 | Author: Jasmin Buijs | Filed under: Food |
 Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
This question stems from proceedings initiated by the German unfair competition association Verband Sozialer Wettbewerb e.V.. We owe many ECJ rulings to this German association, including the famous TofuTown ruling from which it follows that milk designations are reserved for animal dairy products only. We covered the TofuTown case in an earlier blogpost. The current case is against the food supplement company Novel Nutriology, which sells, among other things, an ‘anti-stress’ supplement containing saffron and melon juice extract.
The saffron extract is said to create a more positive mood, according to the seller’s website. This expression is backed by the results of an open study on 50 participants over a 30-day period. Research has shown that the melon juice extract reduces feelings of stress and fatigue, according to the website. Verband Sozialer Wettbewerb e. V. considers these to be unpermitted health claims. It therefore requested Novel Nutriology to stop making these claims, but the supplement company did not listen.
Approved health claims
Health claims are in principle only allowed if they are included in the lists of approved claims under the EU Claims Regulation. This follows from article 10(1) Claims Regulation. References to general, non-specific health benefits such as ‘heart health’ and ‘mental energy’ are allowed if accompanied by a specific, approved claim (article 10(3) Claims Regulation). However, health claims for botanicals are ‘on hold’ and are therefore not included in the lists of approved claims. Therefore, the literal text of Article 10(1) and (3) Claims Regulation cannot be met when making health claims for botanicals.
Claims Regulation nevertheless applicable to botanicals?
To determine whether Novel Nutriology violates the prohibition on making unauthorized health claims, it is essential to know whether Article 10(1) and (3) of the Claims Regulation apply to botanicals. If not, violation of these provisions is out of the question.
As can be seen from the summary of the request for the preliminary ruling, the view is generally taken that references to general health benefits of botanicals must comply with Article 10(3) Claims Regulation. The requirements of this provision are met if the generic health benefit is accompanied by the full, specific health claim that is ‘on hold’. That full ‘on hold’ claim, although not officially authorized, may be used in accordance with the transitional measures in Article 28(5) and (6) Claims Regulation. It would not be compatible with the purpose of Article 10(1) and (3) Claims Regulation to exclude these provisions entirely for botanicals. Such would namely mean that botanical substances may be advertised with non-specific health claims without a scientific assessment of the specific claim supporting them.
Alternative views
Nevertheless, the Bundesgerichtshof finds it unclear whether Article 10(1) and (3) Claims Regulation apply to botanicals. As an argument against applicability, the German court states that the Union legislator would have considered an absolute ban on general health benefits as too broad. Therefore, the Union legislator intended to ban such only if the general claim is not accompanied by an approved, specific health claim.
It is currently however impossible to obtain approval for botanical claims that are ‘on hold’ due to the European Commission’s inaction. By making Article 10(3) Claims Regulation applicable to these substances, the prohibition becomes broader than the Union legislator would have intended. It should therefore be assumed that general health benefits for botanicals are not regulated until the European Commission continues the authorization procedure for ‘on hold’ claims. Based on this alternative view, Article 10(3) of the Claims Regulation should not apply until then.
A second argument raised against the applicability of Article 10(1) and (3) Claims Regulation to botanicals is that the European Commission has not taken action on ‘on hold’ claims for many years. Upholding the applicability of Article 10(1) and (3) Claims Regulation, which cannot be met for ‘on hold’ claims, would lead to a disproportionate restriction of the interests of companies making such claims.
Previous ECJ rulings
 Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Aforementioned report also highlights that the ECJ previously made explicit that Article 28(5) Claims Regulation makes an exception for the use of specific health claims as referred to in Article 13(1)(a) Claims Regulation that have not yet been officially approved. The report therefore concludes that the mentioned transitional regime only applies to the full specific claim and not to general health benefits. General health benefits would therefore not be allowed, although it is recognized that some EU member states do accept general health benefits for botanicals when accompanied by the full ‘on hold’ claim.
Relevance for practice
Health claims for botanicals are currently accepted in the Netherlands if the roadmap of the Dutch agency regulating health products (the Keuringsraad) in cooperation with the NVWA, is met (see here, in Dutch). In brief, this means that (i) the substance and claim are present on the ‘on hold’ list, (ii) potential conditions of use such as a daily dosage are included on the label, (iii) the claim is in line with the wording accepted on KOAG KAG’s ‘indicative list’, and (iv) a disclaimer regarding the ongoing approval procedure is made.
If the ECJ rules that Article 10(1) and (3) Claims Regulation does not apply to botanicals, it is uncertain whether the Keuringsraad roadmap can be upheld. It is however more likely that the ECJ will clarify that botanicals do fall within the scope of the aforementioned provisions. In that case, various outcomes are still possible. In connection with the transitional provisions of Article 28 Claims Regulation, a difference may arise for full ‘on hold’ claims and general health benefits. Such will partly depend on whether the ECJ sticks to the literal text of the transitional provisions (which would prohibit the use of general health benefits), or takes into account current practices (in which case advertising with general health benefits remains allowed under certain conditions). To be continued!
Posted: April 21, 2023 | Author: Jasmin Buijs | Filed under: Authors, Food, Information |
 The ‘farm to fork’ strategy calls for better communication to consumers about healthy and sustainable foods. To make this happen, EU legislation on food information to consumers is currently being revised.
The ‘farm to fork’ strategy calls for better communication to consumers about healthy and sustainable foods. To make this happen, EU legislation on food information to consumers is currently being revised.
The revision of the FIC Regulation covers front-of-pack nutrition labeling, establishment of nutrient profiles, origin labeling and date marking (‘best before’ / ‘use by’). The revision as prompted by the ‘farm-to-table’ strategy further relates to the labeling of alcoholic beverages, as announced in the European Cancer Control Plan.
Need for change
Our daily nutritional intake in Europe is not in line with national and international dietary recommendations. This leads to diet-related chronic diseases, such as diabetes and cardiovascular disease, with all kinds of consequences. According to the European Commission, this is partly because current labeling rules do not provide sufficient guidance for consumers to choose healthy foods.
Various proposals
As part of the legislative process, the European Commission published a so-called ‘Impact Assessment‘ at the end of 2020. The Impact Assessment sets out the labeling rules that need adjustment, and the different options for amendment as proposed by the European Commission. The Impact Assessment for tightened regulations on alcoholic beverages labeling was published later, in the summer of 2021. Below we discuss which changes to the FIC Regulation are proposed in the Impact Assessments and why, and touch upon the options presented for each topic. Later this year, the European Commission will follow up with a proposal for an amended regulation.
FOP labeling
Consumers do not always understand the nutritional information on packaging, which makes it difficult to choose healthy foods. To help consumers making better decisions, there are all kinds of voluntary initiatives for clear front-of-pack (FOP) nutrition labeling. Examples include the much discussed Nutri-Score, but also other initiatives such as the traffic light system (UK), the keyhole (Scandinavia) and the battery (Italy). However, these diverse FOP logos do not necessarily help European consumers as they do not provide equal access to information. The European Commission is concerned that this could lead to fragmentation of the single market, costs for food companies operating in several member states, and confusion among consumers. Harmonizing FOP logos and making them mandatory or not, are options on the table.
 Nutrient profiles
Nutrient profiles
Another proposed change concerns nutrition and health claims on unhealthy products. For example, consider the claim “source of fiber” for biscuits and “rich in vitamin C” for soft drinks. Such claims obscure the unhealthy profiles of these products, which leads to ‘health washing’ (term as introduced by the Dutch consumer association, see here). Nutrient profiles, i.e. thresholds for fats, sugars and/or salt above which the use of nutrition or health claims is restricted, could provide a solution to this. Such thresholds could help preventing consumer deception and create a level playing field for food companies. According to the Claims Regulation, nutrient profiles should already have been established as early as 2009, but this never materialized due to intense debate on the matter. The revision of the FIC Regulation revitalized this discussion. Based on stakeholder feedback on the Impact Assessment, the time seems ripe for change now.
Origin labeling
There is a growing demand from consumers to know the origin of food products. This allows consumers to make more sustainable choices, such as by choosing local products. Origin labeling is already mandatory for certain types of meat, among others. In the absence of harmonized rules regarding other food categories, a number of member states developed national rules for this purpose. Rules differentiating from member state to member state however lead to unequal access to information within the EU and fragments the internal market. The European Commission is therefore investigating whether and, if so, how, European rules on origin labeling could be further extended. This could mean an expansion of the product groups for which origin indication is required. Current discussions also include the production phase to which the indication should refer and the area size referred to (EU / non-EU, or e.g. country or regional level).
Date marking
We currently have two types of date marking. Whereas ‘use by’ refers to the expiry date for food safety reasons, ‘best before’ refers to the date by which the food retains its optimum quality. After this date, color variations may occur, for example, but the product is still safe to eat. The Impact Assessment states that less than one in two consumers understand the meaning of the two date markings. As a result, a lot of food ends up in the waste bin unnecessarily. By estimate 10% of the 88 million tons of food that is wasted annually is linked to date marking. In the context of improving sustainability by reducing food waste, the Impact Assessment presents options to educate consumers about date marking. It also suggests the possibility of removing the ‘best before’ quality date where this has little or no added value.
 Labeling of alcoholic beverages
Labeling of alcoholic beverages
Health damage from alcohol is a serious public health issue in the EU. To reduce alcohol consumption, it is important to inform consumers about what is actually in alcoholic beverages, both in terms of ingredients and nutrients. Currently, this is not mandatory for alcoholic beverages with an alcohol volume above 1.2%. Having said that, there are various initiatives for better information provision on alcohol, such as self-regulation within the beer and spirits sectors. To create a level playing field for operators, the European Commission communicated among others a proposal to remove the aforementioned labeling exception for all alcoholic beverages. Some of the then required information could be communicated off-label via a QR code.
Follow-up steps
Interested parties were able to share feedback with the European Commission after the publication of the two Impact Assessments. To further prepare the legislative proposal, a public consultation took place from 13 December 2021 to 7 March 2022. This process was designed to gather further views, experiences and suggestions on food labeling from stakeholders. As many as 3224 citizens, companies, interest groups, public authorities and other parties filled out the questionnaire made available for this purpose. A large proportion of respondents expressed their support for a harmonized FOP logo, as well as for improved terminology or a visual presentation of date marking. The European Commission’s legislative proposal is expected later this year.
This blogpost has also been published in Dutch at VMT.nl. The author thanks Marie-Claire Evers for her translation of this blogpost into English.
Posted: January 3, 2023 | Author: Jasmin Buijs | Filed under: Authors, Food |
 Last October, AXON contributed to the 16th European Food and Feed Law Conference by a session on circular economy, waste, packaging law, alternative materials, and the Single-Use Plastics (SUP) Directive. While we were back then still waiting for proposals by the European Commission on packaging (waste) and bioplastics, these long-awaited proposals have now been published. This blogpost discusses the main take-aways from these recent European proposals and provides deeplinks to the texts involved.
Last October, AXON contributed to the 16th European Food and Feed Law Conference by a session on circular economy, waste, packaging law, alternative materials, and the Single-Use Plastics (SUP) Directive. While we were back then still waiting for proposals by the European Commission on packaging (waste) and bioplastics, these long-awaited proposals have now been published. This blogpost discusses the main take-aways from these recent European proposals and provides deeplinks to the texts involved.
Packaging problem
Packaging plays without a doubt a very important role in the placing on the market of food. It protects and preserves food, and therefore contributes to increased shelf life and reduced amounts of food waste. It also offers a way to communicate food information to the consumer. At the same time, packaging, just like anything else we create, leaves an impact on the environment. As communicated in the Green Deal, it is the EU’s ambition to lower our amount of packaging waste as part of the green transition.
Bio-plastics
In a search to meet the EU’s goals for a circular economy and climate-neutrality by 2050, bio-plastics are emerging on the market as alternatives for conventional plastics. Bio-plastics can bring several advantages such as making packaging production less dependent on fossil fuels and reducing litter as they may dissolve over time. Their use is however not without challenges as discussed in an earlier blogpost. To improve the understanding around these materials and to clarify where bio-plastics can bring genuine environmental benefits, the European Commission recently published a Communication towards other EU bodies on a policy framework for bio-plastics. As is already clear from existing rules on environmental claims, the Commission stresses that generic claims on packaging such as ‘bio-plastic’ should be avoided to stay away from greenwashing and misleading consumers. It furthermore proposes to only label bio-plastic packaging and other products as ‘biobased’, ‘biodegradable’ or ‘compostable’ when it meets certain conditions. The main take-aways for businesses are as follows:
dissolve over time. Their use is however not without challenges as discussed in an earlier blogpost. To improve the understanding around these materials and to clarify where bio-plastics can bring genuine environmental benefits, the European Commission recently published a Communication towards other EU bodies on a policy framework for bio-plastics. As is already clear from existing rules on environmental claims, the Commission stresses that generic claims on packaging such as ‘bio-plastic’ should be avoided to stay away from greenwashing and misleading consumers. It furthermore proposes to only label bio-plastic packaging and other products as ‘biobased’, ‘biodegradable’ or ‘compostable’ when it meets certain conditions. The main take-aways for businesses are as follows:
Biobased:
- specify the exact and measurable share of biobased plastic content in the packaging; and
- ensure that the biomass is sustainably sourced – priority should be given to the use of organic waste or by-products rather than to primary biomass.
Biodegradable:
- specify that biodegradable packaging should not be littered;
- do not label products covered under the SUP Directive (the scope of which we discussed in more detail here and here) and other short-lived applications and/or litter-prone packaging as biodegradable; and
- specify how long the product needs to biodegrade, under which circumstances and in what environment.
Compostable:
- label only industrially compostable plastics that comply with relevant standards – the Commission will request the revision of the European Standard EN 13432:2000 for this purpose;
- use industrially compostable plastics only if the environmental benefits are higher than their alternatives and if they do not have a negative impact on the quality of the compost, taking into account consumer behavior; and
- specify the way in which the packaging should be disposed of using pictograms.
The Communication on bio-plastics refers, where relevant, to the Commission proposal for a Packaging and Packaging Waste Regulation (PPWR). The aforementioned proposal was also published at the end of last year and is discussed next.
Packaging and Packaging Waste Regulation
Before diving into the Commission’s proposal for a PPWR, it is useful to provide some background on the legal framework for packaging waste as guided by the Waste Framework Directive (WFD).
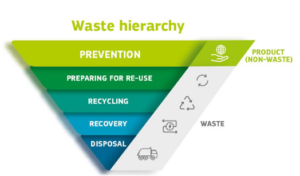 The WFD (currently under revision) introduces the waste hierarchy for waste management, which establishes an order of the preferred disposing route. Waste should in the first place be prevented/reduced. If this is not possible, re-use and thereafter recycling options should be looked into. Only in case this is (or is no longer) an option, energy recovery through incineration or ultimately landfill disposal should be considered. To reduce waste, the WFD also introduces the polluter pays principle and the extended producer responsibility, based on which the waste producer bears financial and/or organizational responsibility for the management of the waste stage at the end of a product’s life cycle.
The WFD (currently under revision) introduces the waste hierarchy for waste management, which establishes an order of the preferred disposing route. Waste should in the first place be prevented/reduced. If this is not possible, re-use and thereafter recycling options should be looked into. Only in case this is (or is no longer) an option, energy recovery through incineration or ultimately landfill disposal should be considered. To reduce waste, the WFD also introduces the polluter pays principle and the extended producer responsibility, based on which the waste producer bears financial and/or organizational responsibility for the management of the waste stage at the end of a product’s life cycle.
The proposal for the PPWR is the Commission’s answer to the revision of the current Packaging and Packaging Waste Directive (PPWD), which focuses on reducing, re-using and recycling packaging. The choice of legal instrument (a regulation rather than a directive) should facilitate a harmonized approach across the various EU Member States. The Regulation is however not an easy read. Although it contains only 65 legal articles, it includes many exceptions to the measures it proposes. For companies that want to get a feel of what to expect, we therefore compiled a list of the most important topics addressed in the Commission’s proposal for the PPWR.
- Requirements for packaging to be recyclable. From 2030, all packaging will have to be ‘designed for recycling’ in accordance with state-of-the-art collection, sorting and recycling processes. As of 2035, packaging must be ‘recycled at scale’, meaning that packaging must be sufficiently and effectively collected, sorted and recycled in practice. Further details on the design for recycling and recycling at scale requirements shall follow by delegated acts adopted by the Commission.
- Minimum amount of recycled plastic content. From 2030, plastic packaging shall contain certain minimum amounts (depending on the type of packaging) of recycled plastic content. These amounts shall further increase by 2040. Instructions as to the methodology for the calculation and verification of the percentage of recycled content will follow by an implementing act. Where information on the recycled content is communicated on the packaging, harmonized labels shall be used for such.
- Mandatory industrial composability for certain packaging. Think of coffee and tea bags or other units, sticky labels attached to fruit and vegetables, and very lightweight plastic carrier bags. The list of packaging that need to be industrially compostable may be extended in future. Packaging that could have been designed as re-usable shall not be presented as compostable.
- Sorting instructions. Labels with information on the material composition shall be applied on packaging to help consumers identifying the appropriate disposal route.
- Rules on re-use and refill. Certain economic operators in the take-away and beverage sector will be subject to targets on re-use and refill. Re-usable packaging must fulfill a set of criteria, including being part of a system for re-use. Information facilitating re-use must be provided on the packaging via a QR code or otherwise. In case of products offered through refill, end-consumers shall be provided with information to ensure safe and hygienic use of the product.
- Increased rules for manufacturers to demonstrate compliance. Manufacturers manufacturing packaging under their own name or trademark, or having packaging designed or manufactured for use with their products, are subject to increased rules to demonstrate compliance with the PPWR. Where a manufacturer is supplied with packaging or packaging materials from a third party, such supplier must provide the manufacturer with all information necessary to demonstrate conformity.
- New roles of economic operators. A system of checks & balances is introduced by giving authorized representatives, importers and distributors specific verification tasks to ensure packaging is placed on the market in accordance with the PPWR.
- Harmonized criteria for modulated extended producer responsibility fees. Financial contributions to be paid by producers (those making available packaging for the first time in the EU under their own name of trademark) to take responsibility for the management of packaging at their end-of-life shall be modulated based on the recyclability of the packaging and the presence of recycled plastic content.
- Reduced empty space in e-commerce and other packaging. The weight and volume of packaging shall be minimized as much as possible. The ratio of empty space in e-commerce and other pre-defined packaging in relation to the packaged product(s) shall not exceed 40%.
- Prohibition on packaging in certain formats and for certain purposes. This mainly concerns certain single-use applications such as in the HORECA sector and for small amounts of fresh fruit and vegetables. The list of prohibited packaging is presented in Annex V to the proposed PPWR and can be amended by delegated act.
- A deposit-return system for single-use plastic beverage bottles and beverage cans. Where these systems do not yet exist for packaging up to 3 liters, such shall be implemented by 2029. For other packaging, Member States are encouraged to voluntarily set up deposit-return or other systems to enable re-use or refill.
Take-away for businesses
As follows from the above, EU food packaging legislation is currently under revision: sustainable packaging will be the new norm. Although the Communication is not and the proposal for the PPWR is not yet binding law, companies involved with food packaging are advised to take the published information serious and to prepare for the enactment of official legislation. This does not only mean staying up-to-date with upcoming legal requirements applicable to the business at stake, but also being ready to involve and control partners in the supply chain through (revised) legal contracts.
Posted: December 1, 2022 | Author: Jasmin Buijs | Filed under: Advertising, Enforcement, Food, Food Supplements, Health claims |
Intro
Food businesses operators that make medical claims for their products in the Netherlands can be fined for doing so under food law. However, they also run the risk of being fined under the Dutch Medicines Act (in Dutch: “Geneesmiddelenwet”), in which case much higher fine amounts apply. The latter sometimes provokes surprise and outrage. Based on three recent rulings, we see a positive trend, which is explained below.
Drug definition
 In principle, a medicinal product cannot be sold in the Netherlands without an authorization. Advertising a medicinal product that has not been authorized is prohibited as well. If a product is classified as such and sold without a license, the seller risks a hefty fine.
In principle, a medicinal product cannot be sold in the Netherlands without an authorization. Advertising a medicinal product that has not been authorized is prohibited as well. If a product is classified as such and sold without a license, the seller risks a hefty fine.
The legal definition of the term medicinal product and the corresponding authorization requirement can be found in the Dutch Medicines Act, which is based on the European Directive 2001/83/EC (the “Medicinal Product Directive”). The Medicinal Product Directive provides two criteria for the definition of a medicinal product: qualification by presentation and qualification by function. If a product meets one of these two criteria, it is classified as a medicinal product. The aforementioned criteria are further elaborated in case law.
Qualification by function
A product is a medicinal product by function (see the Hecht-Pharma judgment) if it can be administered to cure or prevent disease, diagnose or otherwise affect a person’s bodily functions. Of particular importance here are the composition and properties of the product, the method of use, the extent of distribution of the product, the consumers’ familiarity with the product and the health risks associated with its use.
Qualification by presentation
When applying the presentation criterion (see the Van Bennekom judgment), consideration is – unsurprisingly – given to whether a product should be regarded as a medicinal product on the basis of its presentation. It is not necessary that the product is expressly indicated or recommended as a medicinal product. The presentation criterion is already met if the manner of presentation gives the average consumer the impression that the product has a medicinal effect. The form in which the product is presented may give an indication for this, especially in the case of tablets, pills and capsules.
In particular the presentation criterion poses a risk to food companies. If they (unintentionally) make a medical claim in respect of their product, the presentation criterion may result in this product being classified (also) as a medicinal product by the Dutch Food and Consumer Product Safety Authority (“NVWA”). In that case, the NVWA may issue a fine under the Dutch Medicines Act. The starting point for such fine is €150,000, which is then differentiated based on the Policy rules of the Dutch Ministry of Health 2019. Even if the product also falls within the legal definition of food, the Dutch Medicines Act may apply simultaneously. The foregoing follows from the so-called hierarchy provision embodied in article 2.2 of the Medicinal Products Directive, which has been implemented into Dutch law as well. On the basis of this hierarchy principle, the Dutch Medicines Act is applicable if there is any doubt about the applicable product category. The result of this provision is that even a seller of coconut oil can receive a fine under the Dutch Medicines Act.
New trend in enforcement of medical claims?
Dutch case law gives numerous examples of products being classified as medicinal products by courts based on (solely) the presentation criterion. Recently, three court rulings have been rendered which give reason to assume that there is a new trend in case law. These are a ruling of the District Court of Oost-Brabant of March 25, 2022, and two (materially identical) rulings of the District Court of The Hague of June 28, 2022, regarding food supplements and follow-on milk, respectively.
The first case concerns the sale of dietary supplements, for which medical claims were made. The NVWA therefore classifies these supplements as medicinal products based on the presentation criterion and imposes two fines under the Dutch Medicines Act (both for sale and for advertising an unregistered medicinal product). The seller’s defense is that the Dutch Medicines Act should be interpreted in accordance with the Medicinal Product Directive and that it follows from there that the contested decision of the NVWA is based on an incorrect legal basis.
 The court agreed with this argumentation, referring to the amendment of the Medicinal Product Directive of 2004. The court deduces from the preamble to the amendment that the Medicinal Product Directive does not apply if there is no doubt that a product clearly exclusively belongs to another product category, such as food or food supplements. The court ruled that this was indeed the case for the specific circumstances that were under discussion. The products clearly fall under the category of food supplements and therefore solely food law applies. The court confirms that the Dutch Medicines Act must, after all, be interpreted in accordance with the Medicinal Product Directive. The court therefore does not proceed testing the medical claims made against the presentation criterion based on drug legislation at all.
The court agreed with this argumentation, referring to the amendment of the Medicinal Product Directive of 2004. The court deduces from the preamble to the amendment that the Medicinal Product Directive does not apply if there is no doubt that a product clearly exclusively belongs to another product category, such as food or food supplements. The court ruled that this was indeed the case for the specific circumstances that were under discussion. The products clearly fall under the category of food supplements and therefore solely food law applies. The court confirms that the Dutch Medicines Act must, after all, be interpreted in accordance with the Medicinal Product Directive. The court therefore does not proceed testing the medical claims made against the presentation criterion based on drug legislation at all.
Clearly food-only
The above ruling raises the question when a product is “clearly exclusively” a food and what aspects of the product are important in this respect. Indications for this can be found in the two recent decisions of the District Court of The Hague regarding specific food products for toddlers, namely follow-on formula. In its assessment of whether the follow-on formula in question could be a medicinal product by presentation, the court determined that such qualification is not obvious with regard to products sold in supermarkets and drugstores. Another factor in this case was that the detailed information about the follow-on formula, on the basis of which the Dutch Ministry of Health (the counterparty in the cases at stake) believed it to be a medicinal product by presentation, could only be found on the seller’s website.
Conclusion
Based on the rulings discussed, we signal a trend that judges are halting the current practice of enforcement of prohibited medical claims for food products based on the Dutch Medicines Act. The discussed rulings make clear that (prohibited) health claims for food supplements and for other food products such as follow-on formula should be assessed on the basis of the Food Information for Consumers Regulation (the “FIC Regulation”), and not via the presentation criterion based on the Dutch Medicines Act. In our opinion this is justified, because since the FIC Regulation became applicable, food law is specifically set up to do so. We are very curious to see whether the trend initiated above will be followed by other courts. Although it follows from a ruling of the District Court of Zeeland-West-Brabant of 21 October, 2022, that this is not yet the case, we trust this will only be a matter of time.
The above does however not mean that food business operators would be allowed to make medical claims for their products. Also, the FIC Regulation contains a ban on medical claims for food products and the Nutrition and Health Claims Regulation sets forth a strict regime for authorized health claims. Having said that, fines following a violation of food legislation are far lower than fines based on the Dutch Medicines Act. On balance, food companies are therefore better off with fines based on food legislation.
This blogpost is written by Max Baltussen, Karin Verzijden and Jasmin Buijs.
The authors want to acknowledge Ebba Hoogenraad and Irene Verheijen for sharing the case law discussed here.
Posted: September 1, 2022 | Author: Jasmin Buijs | Filed under: Uncategorized |
Last summer, the European Single-Use Plastics (SUP) Directive became applicable. This Directive places restrictions on the use of single-use plastic food packaging (and other plastic products). Where do we stand 1 year after the SUP Directive’s date of application? How has this Directive been implemented in the Netherlands, what are the bottlenecks and what can we still expect?
 The EU has set itself the goal of becoming a leader in the global fight against marine waste and pollution from plastic products. The SUP Directive contains several measures to help reaching this goal. These range from marking requirements on the presence of plastic and its negative impact on the environment (e.g. on to-go coffee cups) to a total ban for products for which more sustainable alternatives are already available (such as for plastic plates and cutlery). However, the scope of the SUP Directive is not crystal clear. For example, the definition of ‘single-use plastic product’ is interpreted differently in different countries.
The EU has set itself the goal of becoming a leader in the global fight against marine waste and pollution from plastic products. The SUP Directive contains several measures to help reaching this goal. These range from marking requirements on the presence of plastic and its negative impact on the environment (e.g. on to-go coffee cups) to a total ban for products for which more sustainable alternatives are already available (such as for plastic plates and cutlery). However, the scope of the SUP Directive is not crystal clear. For example, the definition of ‘single-use plastic product’ is interpreted differently in different countries.
Scope of the SUP Directive
All types of plastics
The SUP Directive defines plastic as a material consisting of a polymer as defined in the REACH Regulation (and which can function as a main structural component of final products – more about this requirement below). Additives or other substances may have been added to the polymer. Natural polymers that have not been chemically modified are not covered by the definition of plastic in the SUP Directive. While the Directive does not provide a further explanation of this concept, the European Commission’s Guidelines (“EC Guidelines” or “Guidelines”) provide further clarification. The aim of the Guidelines is to ensure a harmonized interpretation of the SUP Directive. According to these Guidelines, practically all types of plastics are covered by the SUP Directive, including so-called bio plastics. The Netherlands follows this interpretation in its Explanatory Memorandum to the Dutch SUP Regulation. What supported this approach by the Dutch government is its intention to prevent a shift from single-use plastic packaging to other types of disposable packaging that may also end up as litter and has a negative impact on the environment.
Even products with a small amount of plastic
Both products that consist wholly and partially of plastic can fall under the scope of the SUP Directive. The Directive does not refer to a certain minimum percentage of plastic that must be present. The EC Guidelines clarify that a qualitative assessment should be made, taking into account the objectives of the Directive. Thus, according to the EC Guidelines, otherwise non-plastic products fall under the definition of plastic as referred to in the SUP-Directive if a plastic layer or coating is applied to provide protection against water or fat. Think, for example, of paper- or cardboard-based cups for beverages. Such products often end up as litter. Continuing to allow these products without restrictions does not fit the transition to a circular economy, as part of which waste must be reduced and reuse encouraged. Having said that, non-plastic products seem to be excluded from the scope of the SUP Directive if these contain polymeric materials solely in the form of paints, inks and adhesives (recital 11 to the SUP Directive).
that must be present. The EC Guidelines clarify that a qualitative assessment should be made, taking into account the objectives of the Directive. Thus, according to the EC Guidelines, otherwise non-plastic products fall under the definition of plastic as referred to in the SUP-Directive if a plastic layer or coating is applied to provide protection against water or fat. Think, for example, of paper- or cardboard-based cups for beverages. Such products often end up as litter. Continuing to allow these products without restrictions does not fit the transition to a circular economy, as part of which waste must be reduced and reuse encouraged. Having said that, non-plastic products seem to be excluded from the scope of the SUP Directive if these contain polymeric materials solely in the form of paints, inks and adhesives (recital 11 to the SUP Directive).
In practice, there is (still) a lot of discussion about the question when a product is or is not a plastic product as referred to in the SUP Directive. Some EU Member States introduced a certain threshold value. As a result, paper and cardboard beverage cups and other food packaging that contain polymers in amounts below the maximum threshold do not fall under the SUP Directive in those Member States. The Netherlands purposely did not introduce such limit. Moreover, the Netherlands considers polymers applied as water or fat barriers to be a structural main component of food packaging. The reasoning behind this is that the packaging does not function for its intended use without such barrier. As a result of this approach, almost every material used for disposable food (incl. beverage) packaging falls within the scope of the Dutch SUP measures. As stated in the above-mentioned Explanatory Memorandum, this facilitates enforcement of the measures and makes them better fraud-proof.
Single-use vs. reuse
Once it has been determined that a product qualifies as a plastic product under the SUP Directive, it is relevant to determine whether the product is intended for single-use or reuse. Obviously, the SUP Directive only applies in case of the former. The mere fact that a product may be sold for multiple use cycles is insufficient to pass this test; relevant is whether reuse is included in the design of the product. For example, can the product be adequately cleaned without creating (food safety) hazards for the expected use? The possible reuse of packaging may be determined on the basis of the essential requirements in the Packaging and Packaging Waste Directive. Packaging being suitability for reuse can also be proven if part of a system that guarantees such reuse. Consider, for example, plastic beverage cups that, after use, are taken back by the dispensing location to be cleaned and then refilled. Whether a product is intended for one-time use must be considered on a case-by-case basis. The Dutch competent authority in the Netherlands, Human Environment and Transport Inspectorate (in Dutch: Inspectie Leefomgeving en Transport, or “ILT”), may issue additional guidelines for this purpose for the Dutch market.
Implementation in the Netherlands
In the Netherlands, the SUP Directive has been implemented through the SUP Decree (in Dutch: “Besluit kunststofproducten voor eenmalig gebruik“). This decree required amendments to the Packaging Management Decree 2014 (in Dutch: “Besluit beheer verpakkingen 2014”). The measures under the Decree are further detailed in the SUP Regulation (in Dutch: “Regeling kunststofproducten voor eenmalig gebruik”). The Dutch SUP Regulation gives substance to the freedom of policy granted to EU Member States under the EU SUP Directive. In concrete terms it sets rules for consumption reduction. For this purpose, different regimes will apply to consumption on-the-go on the one hand, and onsite use of disposables on the other hand. Extended producer responsibility, awareness raising measures (such as through national campaigns) and monitoring and reporting obligations are also covered by the Dutch SUP Regulation. The Regulation knows a phased implementation from January 1, 2023. The Dutch Ministry of Infrastructure and Water Management (in Dutch: Ministerie van Infrastructuur en Waterstaat, or “I&W”) keeps stakeholders informed of SUP-related developments in the Netherlands through online newsletters and webinars. A lot of information about the SUP Directive can also be obtained via the Netherlands Institute for Sustainable Packaging (in Dutch: “Kennisinstituut Duurzaam Verpakken”, or “KIDV”), such as a handy decision tree.
Practice in the Netherlands
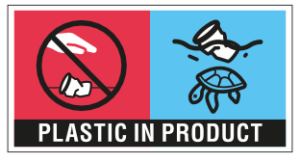 The new rules have not gone unnoticed by consumers. Packaging for food on-the-go and other single-use plastic products now carry a marking that confronts the consumer with their use of such products. Several beverage dispensing locations have already taken a lead on the abovementioned Dutch SUP Regulation and have in place a deposit system for collection and reuse. The above examples show that various producers and importers have taken the necessary measures to act in accordance with the SUP Directive. At the same time, we notice that companies involved in the single-use plastics supply chain still have many questions. In our experience, most of these questions can be answered by carefully reading the detailed EC Guidelines. Since these Guidelines have an authoritative but no legal status, they may however leave the door open for alternative interpretations. So far, we are not aware of any relevant enforcement examples as a result of interpretation disputes. While these may emerge in the future, sufficient enforcement capacity will be a point of attention. Concerns for such are being expressed in Dutch politics, particularly in relation to the upcoming Dutch Single-Use Plastics Regulation. The ILT is currently assessing the required additional enforcement capacity.
The new rules have not gone unnoticed by consumers. Packaging for food on-the-go and other single-use plastic products now carry a marking that confronts the consumer with their use of such products. Several beverage dispensing locations have already taken a lead on the abovementioned Dutch SUP Regulation and have in place a deposit system for collection and reuse. The above examples show that various producers and importers have taken the necessary measures to act in accordance with the SUP Directive. At the same time, we notice that companies involved in the single-use plastics supply chain still have many questions. In our experience, most of these questions can be answered by carefully reading the detailed EC Guidelines. Since these Guidelines have an authoritative but no legal status, they may however leave the door open for alternative interpretations. So far, we are not aware of any relevant enforcement examples as a result of interpretation disputes. While these may emerge in the future, sufficient enforcement capacity will be a point of attention. Concerns for such are being expressed in Dutch politics, particularly in relation to the upcoming Dutch Single-Use Plastics Regulation. The ILT is currently assessing the required additional enforcement capacity.
Differences in Member States
Looking outside our own borders, we notice differences in the implementation of the SUP Directive among EU Member States. For example, some Member States such as Italy apply a threshold value for polymers in non-plastic products (10% by weight), while others don’t. Another example concerns an extended ban on single-use plastic products beyond the products banned under the SUP Directive, such as in France. Consumption reduction measures also vary from Member State to Member State, as shown by the report on reuse systems (in Dutch) commissioned by the Dutch Ministry of Infrastructure and Water Management. As a result of the above, companies that market their products in several Member States face challenges to ensure compliance in all markets in which they are active.
Conclusion
The SUP Directive became applicable over a year ago. The Directive provides for a phased implementation, which we see reflected in the Dutch SUP legislation. The implementation of the SUP-Directive is therefore still in full swing, both at home and abroad. Interpretation and implementation differences are not to be overlooked and companies are challenged to deal with this efficiently. Sharing best practices can be useful here, particularly when the phased implementation progresses and concrete enforcement actions start to take place. Feel free to reach out in case of any implementation issues you want to discuss.
Posted: June 13, 2022 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food, Information, Nutrition claims |
As part of the EU Farm to Fork Strategy for a sustainable food system under the Green Deal, the European Commission agrees that nudging is necessary to guide the consumer to healthier and more sustainable food choices. This has translated into two impact assessments for mandatory front-of-pack (FOP) labeling. Various EU Member States and individual food business operators are however not waiting for harmonized EU FOP labeling and adopted the nutri-score (guiding health choices) and/or eco-score (guiding sustainability choices). This blogpost shows how these initiatives fit into the current and upcoming legal framework.
Nutri-score
 Nutri-score is a five-color nutrition label demonstrating the overall nutritional value of a food product front-of-pack. It allows consumers to compare various foods in a simple and fast way. The label is based on a scale of 5 color and letters, from a dark green “A” for the most healthy choice to a red “E” for the least healthy choice. Simply put, the algorithm behind nutri-score allocates positive points for favorable dietary components (fruits, vegetables, pulses nuts, fibers and proteins) and negative points for energy and unfavorable dietary components (saturated fatty acids, sugars and sodium). The total positive points are subsequently subtracted from the total negative points. The lower the score, the better the letter/color grade.
Nutri-score is a five-color nutrition label demonstrating the overall nutritional value of a food product front-of-pack. It allows consumers to compare various foods in a simple and fast way. The label is based on a scale of 5 color and letters, from a dark green “A” for the most healthy choice to a red “E” for the least healthy choice. Simply put, the algorithm behind nutri-score allocates positive points for favorable dietary components (fruits, vegetables, pulses nuts, fibers and proteins) and negative points for energy and unfavorable dietary components (saturated fatty acids, sugars and sodium). The total positive points are subsequently subtracted from the total negative points. The lower the score, the better the letter/color grade.
Fighting nutrition-related non-communicable diseases
The aim of nutri-score is to nudge the consumer into healthier food choices, and to stimulate the food industry to reformulate their recipes. This way, nutri-score should contribute substantially to a reduced burden of nutrition-related non-communicable diseases such as diabetes, cardiovascular diseases and some types of cancer.
Legal basis
From a legal perspective, nutri-score qualifies as voluntary food labeling in accordance with article 36 FIC Regulation. Food business operators that opt for using nutri-score are however obliged to use it for all foods they place on the market to avoid cherry picking. Moreover, a green “A” or “B” score additionally qualifies as a nutrition claim under the Claims Regulation. Since the claim is not listed in the annex to the Regulation, Member States adopting the nutri-score are subject to the notification procedure of article 23 of the Claims Regulation.
Algorithm changes
Countries that implemented nutri-score (France, Belgium, Luxembourg, Germany and Switzerland) or are willing to use it (Netherlands and Spain) join forces to ensure that nutri-score is in line with the national dietary guidelines. To coordinate such, the abovementioned countries established a Steering Committee and Scientific Committee in February 2021. The Steering Committee is composed of two representatives from national authorities in charge of the nutri-score implementation in each country; the Scientific Committee includes one or two independent experts nominated by each country involved. On March 7 last, the Scientific Committee published its interim report in which it proposes a methodology for modification of the nutri-score algorithm to handle problematic food categories (fats and oils, fish and seafood, whole grain products, salt, sugar, beverages, and dairy products). The Scientific Committee aims at providing a fully revised version of the nutri-score algorithm before the summer. The Steering Committee will have the final say in the recommendations proposed by the Scientific Committee and, where relevant, will elaborate a support document for food business operators to facilitate the appropriation of algorithm changes by the end of the year.
Developments at EU level
In the meantime, the European Commission held a public consultation to introduce standardized mandatory FOP nutrition labeling as part of the revision of the FIC Regulation within the EU’s Farm to Fork strategy. In its impact assessment, it listed five options:
- Baseline (“business as usual”) – it remains possible to voluntarily use a public or private, non-harmonized, FOP label.
- Nutrient- specific labels (numerical) – a harmonized FOP label such as the Italian Nutrinform Battery, providing numerical information on the content of macro nutrients and the energy value of a food, as well as the percentage of the daily refence intake that it makes up for.
- Nutrient-specific labels (color-coded) – a harmonized FOP label such as the UK Multiple Traffic Lights, which is similar to the numerical label but in addition uses colors to classify the content of nutrients as green, amber or red.
- Summary labels (endorsement logos) – a harmonized FOP label such as the Keyhole used in Sweden, which can be applied only to foods that comply with certain beneficial nutritional criteria.
- Summary labels (graded indicators) – a harmonized FOP label such as nutri-score, providing an appreciation of a product’s overall nutritional value through a graded indicator.
The harmonized FOP nutrition label as listed under 2 – 5 above could be either voluntary or mandatory, which is still subject to debate. The impact assessment also mentions the possibility of having a policy mix rather than using one preferred option. Next to the outcome of the public consultation, the European Commission will take into account the comprehensive review on FOP nutrition labeling schemes by the Joint Research Centre (2020) on EFSA’s recent scientific advise on nutrient profiling. A legislative proposal is expected in Q4 2022.
Eco-score
 Eco-score can be seen as the equivalent of nutri-score in the field of sustainability. Just like nutri-score, eco-score is a French initiative. It shows the consumer the environmental impact of a food, using the same presentation as nutri-score in terms of colors and letters. The food’s environmental impact is measured in two steps. First, the environmental footprint is calculated using the Product Environmental Footprint (PEF) method, which is based on a Life Cycle Assessment (LCA). The PEF method takes into account 16 different impact categories, such as ozone depletion, land use and climate change. This eventually translates into a score between 0 and 100. Thereafter, bonus points can be added up or minus points can be deduced from this score. These extra points (positive or negative) are based on 4 additional criteria: (1) food production methods as measured through attributed third-party sustainability credentials such as organic certification, fairtrade or MSC, (2) recyclability of the packaging, (3) the provenance of ingredients, and (4) the stay-away from biodiversity-related issues such as overfishing and deforestation. The LCA takes place at product category level; the allocated bonus and minus points are related to the individual product.
Eco-score can be seen as the equivalent of nutri-score in the field of sustainability. Just like nutri-score, eco-score is a French initiative. It shows the consumer the environmental impact of a food, using the same presentation as nutri-score in terms of colors and letters. The food’s environmental impact is measured in two steps. First, the environmental footprint is calculated using the Product Environmental Footprint (PEF) method, which is based on a Life Cycle Assessment (LCA). The PEF method takes into account 16 different impact categories, such as ozone depletion, land use and climate change. This eventually translates into a score between 0 and 100. Thereafter, bonus points can be added up or minus points can be deduced from this score. These extra points (positive or negative) are based on 4 additional criteria: (1) food production methods as measured through attributed third-party sustainability credentials such as organic certification, fairtrade or MSC, (2) recyclability of the packaging, (3) the provenance of ingredients, and (4) the stay-away from biodiversity-related issues such as overfishing and deforestation. The LCA takes place at product category level; the allocated bonus and minus points are related to the individual product.
Legal basis: currently no specific rules
There is not yet a legal framework specifically dedicated to environmental claims, let alone a legal definition thereof. Environmental claims are currently enforced based on general rules, guidelines and self-regulation within the legal framework of unfair commercial practices and misleading advertisements, as discussed in our earlier blogpost. Interestingly, these different sources produce different definitions of environmental claims. The definition thereof in the Dutch Code for Environmental Advertising is for example very broad and includes the eco-score as a claim related to the environmental factors connected with the product. It is however questionable whether an eco-score “C”, “D” or “E” would fall under the definition of a ‘green claim’ under the EC Guidance on the Unfair Commercial Practices Directive. This latter guidance namely refers to a positive environmental impact (which products with lower scores have not) or a lower damaging impact on the environment than competing products. Since the eco-score algorithm is largely based on product categories rather than individual products, it is not necessarily suitable for comparisons between competing products such as for example different fruit juices. Assuming that eco-score does qualify as an environmental claim, the following question is whether it is in conformity with the applicable rules. These rules are however not black and white and leave room for interpretation, especially since the number of enforcement cases is still rather low.
Future situation: EU harmonization of eco-score?
The ambiguity illustrated above may be over with in the near future, since the European Commission is working towards the harmonized use of a sustainability label under its Farm to Fork Strategy and the transition towards a sustainable food system. In its impact assessment, it lists the following options:
- Baseline (“business as usual”) – No specific new actions, though existing initiatives on environmental claims will be continued, such as the upcoming legislation on the substantiating of environmental footprint claims by use of the PEF or OEF (organizational environmental footprint) method.
- Voluntary approaches – No legislative initiatives but guidance and private initiatives such as codes of conducts.
- Reinforcing existing legislation – Development of sustainability labeling provisions related to more than one sustainability component (such as environmental and social sustainability) through existing sector-specific legislation (for example fisheries marketing standards).
- Voluntary EU sustainability label – Development of a voluntary harmonized sustainability label, either applicable to all foods or to foods that meet a certain sustainability standard only.
- Mandatory EU sustainability label – Development of a mandatory harmonized sustainability label, either for all foods placed on the EU market or mandatory for EU produced foods and voluntary for imported foods.
A legislative proposal is expected in Q4 2023. Until July 21 of this year, it is possible to contribute through the public consultation.
Meanwhile in the Member States….
 Member States seem not to be waiting for the legislative proposals of the European Commission. Instead, various Member States launched national initiatives on FOP sustainability labels and join forces to ensure that such labels will be implemented in a similar way throughout the EU. Taking the Netherlands as an example, the Ministry of Agriculture, Nature and Food Quality aims to implement a voluntary sustainability label for foods in the Netherlands by 2025. This goal forms part of the National Climate Agreement. Together with the food sector and other stakeholders, the Ministry is currently investigating how such a label for the Dutch market could look like. LCA’s based on the PEF-method are taken as a starting point for further development.
Member States seem not to be waiting for the legislative proposals of the European Commission. Instead, various Member States launched national initiatives on FOP sustainability labels and join forces to ensure that such labels will be implemented in a similar way throughout the EU. Taking the Netherlands as an example, the Ministry of Agriculture, Nature and Food Quality aims to implement a voluntary sustainability label for foods in the Netherlands by 2025. This goal forms part of the National Climate Agreement. Together with the food sector and other stakeholders, the Ministry is currently investigating how such a label for the Dutch market could look like. LCA’s based on the PEF-method are taken as a starting point for further development.
Conclusion
FOP labeling is a topic of conversation. Various initiatives on both national and European level are taking place simultaneously, in the hope that they will once come together as an EU harmonized label. We see different food businesses reacting to this situation differently. Where some opt for awaiting formal decisions at national level and instructions by the government, others are pioneering and experimenting with FOP labeling within the currently existing legal framework. Examples include the nutri-score pilot by Iglo and the full-fledged use of eco-score by Colruyt Group in Belgium. What about you? Are you a game changer or a laggerd?
Together with Lisa Gray from Iglo and Veerle Poppe from Colruyt Group, Jasmin Buijs presented this topic at the VMT Food Law Event on June 7 last.
 Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives. Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling. Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims.
Germany’s highest court, the Bundesgerichtshof, asked the European Court of Justice (ECJ) this summer to explain the use of ‘on hold’ claims for so-called ‘botanicals’. The question is whether these substances may be advertised with health claims or general, non-specific health benefits as long as the assessment by EFSA has not been completed and the European Commission has not yet taken a final decision on the authorization of these claims. Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored.
Whether the aforementioned alternative views are sufficient to exclude botanicals from the scope of Article 10(1) and (3) Claims Regulation remains to be seen. As highlighted in a report published last September, companies are able to make health claims for botanicals that are included in the ‘on hold’ list under the transitional regime of Article 28(5) and (6) of the Claims Regulation. Moreover, the ECJ ruled in previous cases that companies making ‘on hold’ claims are not disproportionately disadvantaged. Since they can make claims without EFSA having assessed these and/or without a final decision from the European Commission, they are in fact favored. In principle, a medicinal product cannot be sold in the Netherlands without an authorization. Advertising a medicinal product that has not been authorized is prohibited as well. If a product is classified as such and sold without a license, the seller risks a hefty fine.
In principle, a medicinal product cannot be sold in the Netherlands without an authorization. Advertising a medicinal product that has not been authorized is prohibited as well. If a product is classified as such and sold without a license, the seller risks a hefty fine. The court agreed with this argumentation, referring to the amendment of the Medicinal Product Directive of 2004. The court deduces from the preamble to the amendment that the Medicinal Product Directive does not apply if there is no doubt that a product clearly exclusively belongs to another product category, such as food or food supplements. The court ruled that this was indeed the case for the specific circumstances that were under discussion. The products clearly fall under the category of food supplements and therefore solely food law applies. The court confirms that the Dutch Medicines Act must, after all, be interpreted in accordance with the Medicinal Product Directive. The court therefore does not proceed testing the medical claims made against the presentation criterion based on drug legislation at all.
The court agreed with this argumentation, referring to the amendment of the Medicinal Product Directive of 2004. The court deduces from the preamble to the amendment that the Medicinal Product Directive does not apply if there is no doubt that a product clearly exclusively belongs to another product category, such as food or food supplements. The court ruled that this was indeed the case for the specific circumstances that were under discussion. The products clearly fall under the category of food supplements and therefore solely food law applies. The court confirms that the Dutch Medicines Act must, after all, be interpreted in accordance with the Medicinal Product Directive. The court therefore does not proceed testing the medical claims made against the presentation criterion based on drug legislation at all. The EU has set itself the goal of becoming a leader in the global fight against marine waste and pollution from plastic products. The SUP Directive contains several measures to help reaching this goal. These range from marking requirements on the presence of plastic and its negative impact on the environment (e.g. on to-go coffee cups) to a total ban for products for which more sustainable alternatives are already available (such as for plastic plates and cutlery). However, the scope of the SUP Directive is not crystal clear. For example, the definition of ‘single-use plastic product’ is interpreted differently in different countries.
The EU has set itself the goal of becoming a leader in the global fight against marine waste and pollution from plastic products. The SUP Directive contains several measures to help reaching this goal. These range from marking requirements on the presence of plastic and its negative impact on the environment (e.g. on to-go coffee cups) to a total ban for products for which more sustainable alternatives are already available (such as for plastic plates and cutlery). However, the scope of the SUP Directive is not crystal clear. For example, the definition of ‘single-use plastic product’ is interpreted differently in different countries. that must be present. The EC Guidelines clarify that a qualitative assessment should be made, taking into account the objectives of the Directive. Thus, according to the EC Guidelines, otherwise non-plastic products fall under the definition of plastic as referred to in the SUP-Directive if a plastic layer or coating is applied to provide protection against water or fat. Think, for example, of paper- or cardboard-based cups for beverages. Such products often end up as litter. Continuing to allow these products without restrictions does not fit the transition to a circular economy, as part of which waste must be reduced and reuse encouraged. Having said that, non-plastic products seem to be excluded from the scope of the SUP Directive if these contain polymeric materials solely in the form of paints, inks and adhesives (recital 11 to the SUP Directive).
that must be present. The EC Guidelines clarify that a qualitative assessment should be made, taking into account the objectives of the Directive. Thus, according to the EC Guidelines, otherwise non-plastic products fall under the definition of plastic as referred to in the SUP-Directive if a plastic layer or coating is applied to provide protection against water or fat. Think, for example, of paper- or cardboard-based cups for beverages. Such products often end up as litter. Continuing to allow these products without restrictions does not fit the transition to a circular economy, as part of which waste must be reduced and reuse encouraged. Having said that, non-plastic products seem to be excluded from the scope of the SUP Directive if these contain polymeric materials solely in the form of paints, inks and adhesives (recital 11 to the SUP Directive). The new rules have not gone unnoticed by consumers. Packaging for food on-the-go and other single-use plastic products now carry a marking that confronts the consumer with their use of such products. Several beverage dispensing locations have already taken a lead on the abovementioned Dutch SUP Regulation and have in place a deposit system for collection and reuse. The above examples show that various producers and importers have taken the necessary measures to act in accordance with the SUP Directive. At the same time, we notice that companies involved in the single-use plastics supply chain still have many questions. In our experience, most of these questions can be answered by carefully reading the detailed EC Guidelines. Since these Guidelines have an authoritative but no legal status, they may however leave the door open for alternative interpretations. So far, we are not aware of any relevant enforcement examples as a result of interpretation disputes. While these may emerge in the future, sufficient enforcement capacity will be a point of attention. Concerns for such are being expressed in Dutch politics, particularly in relation to the upcoming Dutch Single-Use Plastics Regulation. The ILT is currently assessing the required additional enforcement capacity.
The new rules have not gone unnoticed by consumers. Packaging for food on-the-go and other single-use plastic products now carry a marking that confronts the consumer with their use of such products. Several beverage dispensing locations have already taken a lead on the abovementioned Dutch SUP Regulation and have in place a deposit system for collection and reuse. The above examples show that various producers and importers have taken the necessary measures to act in accordance with the SUP Directive. At the same time, we notice that companies involved in the single-use plastics supply chain still have many questions. In our experience, most of these questions can be answered by carefully reading the detailed EC Guidelines. Since these Guidelines have an authoritative but no legal status, they may however leave the door open for alternative interpretations. So far, we are not aware of any relevant enforcement examples as a result of interpretation disputes. While these may emerge in the future, sufficient enforcement capacity will be a point of attention. Concerns for such are being expressed in Dutch politics, particularly in relation to the upcoming Dutch Single-Use Plastics Regulation. The ILT is currently assessing the required additional enforcement capacity.












 The WFD (currently under
The WFD (currently under 

