Advocate General opinion reversed: no meaty names ban for plant-based meat substitutes
Posted: October 7, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Authors, clean meat, cultivated meat, Food, Information | Comments Off on Advocate General opinion reversed: no meaty names ban for plant-based meat substitutes On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
On 4 October 2024, the European Court of Justice (ECJ) provided its judgement in the case C-438/23 on the question whether the French national Decree limiting the use of meaty names for plant-based products is in compliance with the Food Information to Consumers Regulation (FIC Regulation). A month earlier, Advocate General (AG) Capeta rendered her opinion in this case, which we analyzed in this blogpost. While this opinion was not very promising for plant-based meat companies, the ECJ did not follow the AG and ruled that no meaty names ban can be implemented at national level. In this blogpost, we explain why, and what this all means for the alternative protein sector. For more background on the French Decree and the preliminary ruling by the ECJ, we refer to our previous blogpost on this topic.
Legal names can be set, but the French Decree does not contain legal names
The ECJ starts the motivation of its decision with acknowledging that the FIC Regulation leaves room to adopt legal names at Member State level where such do not exist at EU level. Where legal names are set, these cannot be used for products not complying with the specifications of that name. As an example, the ECJ refers to the term ‘meat’, which is legally defined as ‘the edible parts of animals’. A food not containing such parts can therefore not use the name ‘meat’, even if it is accompanied by specifying terms such as ‘vegetarian’. The same applies to milk and certain milk products, for which the legal name is laid down in the COM Regulation. Indeed, as we know well from the Tofutown decision, names such as ‘plant-based milk’ are a no-go.
According to AG Capeta, the French Decree under attack established legal names. This was done on the one hand by establishing a list of meaty names of which the use is prohibited for the designation of their plant-based counterpart (such as steak), and on the other hand by authorizing the use of certain meaty names for foods containing vegetable proteins provided that they do not exceed a certain proportion (such as cordon bleu (maximum 3,5 % vegetable protein)).
The ECJ ruled however differently. In the first place, it recalls that the French authorities themselves rejected the hypothesis that Decree No 2022-947 lays down a legal name. Therefore, the learnings from the Tofutown decision cannot be applied to the case at hand. In the second place, it also states that legal names must, according to the definition thereof in the FIC Regulation, be defined in order to designate a foodstuff. The adoption of a legal name thus means associating a specific expression with a given food. This is done by setting certain conditions, especially with regard to the composition of the food. The French Decree contains a measure prohibiting the use of certain meaty names, which are not legally defined by the Decree, for plant-based foods. This is not the same.
Use of customary and descriptive names fully harmonized
Given that there are no legal names for plant-based foods at EU level and neither in France as far as is known to the ECJ based on the file of the case, plant-based foods must be indicated by their customary name or descriptive name. Where a customary name is the accepted name of the food that does not need further explanation, a descriptive name must explain the main characteristics of the food.
 Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
Obviously, Member States cannot prevent plant-based food companies from complying with their obligation under the FIC Regulation to indicate the name of their products by using customary or descriptive names where no legal name exists. Having said that, customary and descriptive names must of course comply with the FIC Regulation and therefore not be misleading in the meaning of art. 7 thereof. As the ECJ indicates, consumers are not easily misled where the substitution of a component or ingredient of a (in this case animal-derived) food is clearly indicated in accordance with art. 7(1)(d) of, and Annex VI, Part A, point 4, to the FIC Regulation. The ECJ motivates that this set of rules also covers the situation where the composition of the food changes completely because the respective component or ingredient constitutes the only component or ingredient of the food (as is the case for e.g. a vegetarian steak). The ECJ therefore concludes that the protection of consumers from the risk of being misled by the use of meaty customary or descriptive names for foods that are fully or partly plant-based is fully harmonized by the FIC Regulation. Therefore, Member State cannot enact national measures regulating or prohibiting the use of such meaty names. The ECJ specifies that this includes that Member States cannot establish maximum permitted levels of vegetable proteins that can be contained in foods to be designated by meaty customary or descriptive names, either.
No distinction between domestic and imported products
Where the AG made in her opinion a distinction between rules covering domestic production and rules covering production abroad, this topic was no longer covered in the ECJ’s preliminary ruling. The question whether the French Decree could only apply to foods manufactured in its territory was initiated by the highest French administrative court (“Conseil d’Etat”), but became redundant since the ECJ came to the conclusion that national measures regulating or prohibiting the use of meaty names for plant-based products (other than by means of legal names) is not allowed in the first place.
We nevertheless conclude from the case that since the limitative legislation applying at a national level is not considered in conformity with Union legislation, this surely goes for national legislation applying similar restrictions on a Union level. This is justified by the fact that the ECJ recalls at the beginning of its decision the two paramount principles of Union legislation. In addition to consumer protection, this is also the smooth functioning of the internal market.
Outlook for the alternative protein sector a whole
Now the ECJ has given its interpretation of the FIC Regulation in response to the questions of the French referring court, it is now up to the latter to decide the dispute at national level in accordance with the ECJ’s preliminary ruling.
It should be noted that the ECJ’s ruling is similarly binding on other national courts or tribunals before which a similar issue is raised. This means that the prohibition to enact national measures regulating or prohibiting the use of meaty names in the absence of legal names in principle also applies to other meat analogues such as cultivated meat and those produced by precision fermentation. Having said that, these products may face other challenges, such as the question whether such products can actually be called meat (for which they must be edible parts of animals as indicated above), and to what extent the production technique used must be indicated in accordance with art. 7(1)(a) of, and Annex VI, Part A, point 1 to, the FIC Regulation. We will keep you posted!
Dutch sequels of Tofutown saga: Thou Shall Not Touch Dairy Names!
Posted: July 25, 2024 | Author: Karin Verzijden | Filed under: Advertising, alternative protein, Authors, Enforcement, Food, Information | Comments Off on Dutch sequels of Tofutown saga: Thou Shall Not Touch Dairy Names! On 23 July 2024 a Dutch Court ruled in summary relief proceedings that Upfield cannot use the name “roombeter” for a plant-based alternative for butter, as this is in violation of the Regulation establishing a common organization of the markets for agricultural products (“COM Regulation”). You should know that “roombeter” translates in English as a composition of “cream” and “better”, whereas “cream” is a reserved designation under the COM Regulation that can only be used for dairy products. Furthermore, “beter” is close to “boter”, being the Dutch designation for butter.
On 23 July 2024 a Dutch Court ruled in summary relief proceedings that Upfield cannot use the name “roombeter” for a plant-based alternative for butter, as this is in violation of the Regulation establishing a common organization of the markets for agricultural products (“COM Regulation”). You should know that “roombeter” translates in English as a composition of “cream” and “better”, whereas “cream” is a reserved designation under the COM Regulation that can only be used for dairy products. Furthermore, “beter” is close to “boter”, being the Dutch designation for butter.
Facts of the case at hand
In the case at hand, Upfield markets a plant-based alternative for butter under the brand BLUE BAND and the product name ROOMBETER. The packaging of the product furthermore states “100 % plant-based alternative for butter” and “81 % less climate impact than butter”. The packaging itself consists of golden coloured paper that is also used for conventional butter in the Netherlands and it displays a curl of butter as shown below. The Dutch Dairy Association opposed the use of the product name ROOMBETER, as it is considered this a violation of the COM Regulation, as explained below.
Case before Dutch Advertisement Code Committee
Prior to this legal procedure , the Dutch Dairy Association had submitted a complaint regarding this product before the Dutch Advertisement Code Committee. This self-regulatory body ruled on March 21 last that the presentation of the product was misleading, since it could be understood to contain butter. The “e” in “beter” could be confused for an “o”, resulting in “boter”, which is Dutch for butter. And furthermore the golden coloured packaging added to the misleading character of the product. The topic of violation of the COM Regulation was left to civil law proceedings, as it exceeded the competence of the Committee.
Applicable legislation
Article 78.2 of the COM Regulation states that the definitions, designations and sales descriptions provided for in its Annex VII may be used in the Union only for the marketing of a product that conforms to the corresponding requirements laid down in that Annex. Annex VII contains, amongst other things, a product definition for milk and a list of milk products. It furthermore states that these designations may not be used for any other product than milk and milk products. The purpose of this provision is to protect dairy names from being used for non-dairy products.
Tofutown
You may recall that in its Tofutown decision back in 2017, the ECJ formulated a very strict prohibition of the use of diary names for non-dairy products (check out our blog on this case here). As a result of that prohibition, the use of the designation “Tofubutter” for a tofu-based product was in violation of the COM Regulation. As a general rule, the ECJ precluded the term ‘milk’ and the designations reserved by the COM Regulation exclusively for milk products from being used to designate a purely plant based product in marketing or advertising. This even applies if those terms are expanded upon by clarifying or descriptive terms indicating the plant origin of the product at issue
Arguments in favour of ROOMBETER
Upfield had argued it did not market its product under the designation ROOMBETER but under the designation BLUE BAND ROOMBETER. The brand BLUE BAND has been used for more than 100 years for margarine, so it is obvious for the consumer this is a plant-based product This is even strengthened by the Dutch translation of the designations “100 % plant-based alternative for butter” and “81 % less climate impact than butter”. So the name ROOMBETER does not designate, imply or suggest it is about a dairy product.
Court decision
The Court did not eat it. Instead, it very strictly applied the Tofutown doctrine, stating that a reserved designation under the COM Regulation cannot be used for a plant-based product. It went on to explain that if it is prohibited to use the designation “tofubutter” for a plant-based product, as it contains the reserved designation “butter”, for sure it is prohibited to use the designation “roombeter” for a plant-based product, as it contains another reserved designation under the COM Regulation. Also, the element “beter” (“better” in English) can hardly be perceived as a clarifying or descriptive term, as it does not refer (contrary to “tofu”) to a plant-based origin. In fact, its reference to plant-based origins can only be understood by those consumers who know the “skip the cow” ad or who further study the packaging of this product.
Upfield was therefore ordered to stop using the designation ROOMBETER within three months after the date of the legal decision.
Consequences of this decision
Should the conclusion of this decision be that any reference to dairy products should be meticulously avoided when marketing plant-based dairy replacements? This seems a very hard task, as manufacturers of these replacement products will want to indicate how their products can be used. Happily, this is not the case. It is still permitted to mention that your plant-based product is for instance a “yoghurt variation”, as this is perceived as a product explanation rather than a product designation. This is not in violation of the Tofutown doctrine and in line with a 2019 Dutch Supreme Court decision relating to a soy-based product marketed by Alpro. Advertising plant-based dairy alternatives nevertheless remains a delicate balancing act.
Advertisement restrictions for formula milk; what about cell-based solutions?
Posted: July 23, 2024 | Author: Jasmin Buijs | Filed under: Advertising, alternative protein, Food, Information | Comments Off on Advertisement restrictions for formula milk; what about cell-based solutions? Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
Claims for formula milk that refer to nature can be understood as a (prohibited) discouragement of breastfeeding, as was recently ruled in two instances by the Dutch Advertising Code Foundation. This case captured our attention, especially with an eye to future possibilities of cell-based breastmilk alternatives.
What is the case about? Advertiser, a food business marketing infant and follow-on formula, published statements on its website about its products such as:
- “Unique combination of natural lactic acid cultures and valuable fibers.”
- “Where organic ends, […] continues: […] guarantees an excellent quality and supersedes the legal requirements concerning organic.”
- “Inspired by nature.”
- “With the first milk that is non mom’s, I want to do all well” (next to a picture of a baby in the arms of a woman).
The question is whether (a) these texts qualify as advertisements, and (b) whether the texts give the impression that the follow-on formula is as good as breastmilk.
What is the legal rule? Consumer-oriented advertising for infant formula is prohibited. Advertisements for follow-on formula are possible, but should not dissuade or discourage breastfeeding. This is laid down in articles 4.1 and 5.2 of the Dutch Advertising Code for Infant Formula, in line with EU Regulation 2016/127. As follows from article 6(6) of aforementioned Regulation, the labelling, presentation and advertising of infant and follow-on formula shall not include terms like ‘humanized’, ‘maternalized’ or ‘adapted’. The Dutch Advertising Code for Infant Formula further lists ‘inspired by breastmilk’, ‘protected effect of breastmilk’, and ‘contains nutrients that are also found in breastmilk’ as examples of prohibited statements to avoid discouragement of breastfeeding.
What was decided? The Board of Appeal upheld the decision of the Advertising Code Committee and ruled that (a) the texts on advertiser’s website are clearly promotional in nature, and that (b) the consumer will understand the references to nature to be references to breastmilk (and not to the products having organic qualities, as was explained by advertiser). As this is in violation of the Dutch Advertising Code for Infant Formula, advertiser is requested to no longer make such advertisements.
 Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Our analysis and future outlook: It is commonly agreed that breastfeeding, where possible, should be supported. This is one of the principles laid down in the WHO International Code of Marketing of Breast-Milk Substitutes and the subsequent relevant resolutions of the World Health Assembly. We are therefore not surprised by the ruling.
Looking into the future, it is interesting to note that the same rules shall in principle apply to cultivated breastmilk made in a lab. Various companies are already working on this concept such as French Nūmi and BIOMILQ in the US. Giants like Danone and FrieslandCampina have announced strategic partnerships with cell-based human milk component start-ups. Companies working on such substitute products obviously want to (and should) explain their products to the public. Statements such as ‘inspired by breastmilk’ and ‘contains nutrients that are also found in breastmilk’ may not seem unreasonable in this context. Currently this is however not allowed. Will the Dutch self-regulatory code (which is more specific in prohibited terms than EU Regulation 2016/127 and the WHO Code) be updated, so that such innovations can be appropriately explained to the public? Stay tuned – we will keep you posted!
The full case can be read here (in Dutch).
European harmonization probiotic claims possible? Here’s the latest.
Posted: April 24, 2024 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food, Food Supplements, Health claims, Information, Nutrition claims | Comments Off on European harmonization probiotic claims possible? Here’s the latest. The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
The International Probiotics Association Europe (IPA Europe) is calling for harmonized use of the claim ‘probiotics’ in the EU. Aforementioned term is generally considered an unauthorized health claim under the EU Claims Regulation. Nevertheless, an increasing number of EU member states allows the use of this claim under certain conditions. This blogpost dives into the regulatory status of probiotic claims in different EU member states and the latest developments in this area.
When entering the keyword ‘probiotic’ in the EU Health Claims Register, one is confronted with over 100 rejected health claims. Over the past years, this has led to the question whether the term ‘probiotic’ should be allowed under certain conditions, and has resulted into divergent policies in different EU member states. To protect both the food industry (against unfair competition) and the consumer (against misleading information), these divergent policies are reason for IPA Europe to call for a harmonized framework.
Czech Republic, Northern Ireland and France
Although EU member states generally consider ‘probiotics’ to be an unauthorized health claim under the EU Claims Regulation, some EU member states take a different approach. ‘Probiotics’ is for example considered a nutrition claim in the Czech Republic. Such claim is allowed if the conditions set forth in the EU Claims Regulation are met. This means, amongst others, that the good bacteria in question are present in the food in an amount that, according to generally accepted scientific evidence, causes the claimed beneficial effect. By contrast, in France and Northern Ireland, the term ‘probiotics’ can be used as a general, non-specific health claim that is allowed in combination with the authorized health claim “live cultures in yoghurt or fermented milk improve lactose digestion of the product in individuals who have difficulty digesting lactose”.
Italy
Italy takes the view that the term ‘probiotics’ does not meet the definition of a health claim and therefore falls outside the scope of the EU Claims Regulation. Italy supports this view by EFSA’s conclusion that probiotic colonization in the intestinal flora (without further specification of bacterial species or strains) is insufficient evidence to substantiate a beneficial effect on human health. In other words, no link between probiotics and health can be demonstrated, for which reason ‘probiotics’ cannot be considered a health claim. Aforementioned reasoning is however not a free pass to unconditionally use the term ‘probiotics’ in Italy. Instead, the following conditions must be met if and when using this term: (i) safety for human consumption, (ii) a history of use for the benefit of the intestinal flora, and (iii) presence of the relevant bacteria in the food in live form and in an adequate quantity until end of shelf life.
Denmark and Spain
In Denmark, the term ‘probiotics’ can be used based on a different legal ground, namely as a mandatory category designation under the EU Food Supplements Directive. In France, such is also possible. Indeed, Article 6(3)(a) of the EU Food Supplements Directive requires that the labeling of the food supplement shall bear “the names of the categories of nutrients or substances that characterize the product or an indication of the nature of those nutrients or substances”. In Spain, the claim ‘probiotics’ is allowed thanks to the principle of mutual recognition. Based on this principle, a product lawfully marketed in one EU member state must also be accepted on the market of another EU member state. Since probiotic claims are therefore allowed on the Spanish market for products from other EU member states, banning the term ‘probiotics’ at national level would discriminate against national producers. Therefore, both food products produced within Spain and those produced outside of the country can bear the claim ‘probiotics’.
Netherlands
In the Netherlands – just like in Denmark and France – the term ‘probiotics’ can be used as a category designation for food supplements. This Dutch practice is laid down in the Guideline Document on the EU Claims Regulation by the Dutch Health Advertising Knowledge and Advice Council (in Dutch: Keuringsraad). An earlier version of the Nutrition and Health Claims Manual by the Dutch Food Safety Authority (in Dutch: NVWA Handboek Voedings- en Gezondheidsclaims) also explicitly mentioned this possibility. Such is now longer mentioned in the latest version of the Nutrition and Health Claims Manual, which can be explained by the fact that ‘probiotics’ as a category designation for food supplements is not a nutrition or health claim.
Although claims that further elaborate on the health effect of probiotics (sporadically) occur on the Dutch (online) market, these are not allowed. Whether the expression “increases the good bacteria in the intestinal flora” (without using the term ‘probiotics’) is acceptable in the Netherlands, is yet unclear. Yakult uses this expression to advertise its fermented milk drink. The Dutch Health Advertising Knowledge and Advice Council would not allow this expression in the context of its preventive supervision in the context of food supplements, because this expression in effect creates a link between the food product and health. If the NVWA has however a different opinion on this and does consider aforementioned expression possible, then other food businesses can take advantage of this too. The call for harmonized uses of the term ‘probiotics’ by IPA Europe, about which more below, can possibly contribute to the acceptance of such expression at national level.
”Probiotics’ not a health claim”
As demonstrated above, various EU member states have introduced national rules on the use of the term ‘probiotics’. As a result, IPA Europe believes that the European Commission’s position that ‘probiotics’ implies a health benefit and is therefore a(n unauthorized) health claim no longer holds water. Instead, IPA Europe advocates qualifying the claim ‘contains probiotics’ as a nutrition claim, just like ‘contains vitamins and minerals’ and ‘contains fiber’. Aforementioned substances may have beneficial nutritional properties, but no specific health benefit is claimed. To strengthen its argument that ‘probiotics’ is not a health claim, IPA Europe explains that the term is not sufficiently precise to substantiate the claimed health benefit under reference to an EFSA guidance document published in 2016.
Call for harmonized use of the term ‘probiotics’
According to IPA Europe, ‘probiotics’ is therefore not a health claim and does not require authorization under the EU Claims Regulation. At the same time, it emphasizes the need for clear rules on the use of the claim as this contributes to a fair competitive environment for food businesses and helps consumers to make informed choices. In December 2023, IPA Europe therefore called for a clearly defined framework for the use of the term ‘probiotics’ in the EU. IPA Europe recommends the following four criteria for consistent use thereof:
- characterization of the species level and identification of at strain level;
- the probiotic strain must be safe for the intended use, e.g. based on the QPS list;
- the probiotic status should be scientifically documented; and
- the probiotic strains must be alive in the product and in a sufficient amount up to the end of shelf-life.
Final comment
The EU knows a fragmented regulatory landscape when it comes to the use of the term ‘probiotics’. Despite IPA Europe’s efforts for a harmonized approach throughout the EU, we will need European legislation or a ruling from the European Court of Justice to have the same rules in all EU member states. For now, the term ‘probiotics’ is (fortunately) not completely banned in the Netherlands and neither in quite a few other EU Member States.
This blogpost has also been published in Dutch at VMT.nl.
The FIC Regulation is due for renewal: How consumers can make healthy and sustainable food choices
Posted: April 21, 2023 | Author: Jasmin Buijs | Filed under: Authors, Food, Information | Comments Off on The FIC Regulation is due for renewal: How consumers can make healthy and sustainable food choices The ‘farm to fork’ strategy calls for better communication to consumers about healthy and sustainable foods. To make this happen, EU legislation on food information to consumers is currently being revised.
The ‘farm to fork’ strategy calls for better communication to consumers about healthy and sustainable foods. To make this happen, EU legislation on food information to consumers is currently being revised.
The revision of the FIC Regulation covers front-of-pack nutrition labeling, establishment of nutrient profiles, origin labeling and date marking (‘best before’ / ‘use by’). The revision as prompted by the ‘farm-to-table’ strategy further relates to the labeling of alcoholic beverages, as announced in the European Cancer Control Plan.
Need for change
Our daily nutritional intake in Europe is not in line with national and international dietary recommendations. This leads to diet-related chronic diseases, such as diabetes and cardiovascular disease, with all kinds of consequences. According to the European Commission, this is partly because current labeling rules do not provide sufficient guidance for consumers to choose healthy foods.
Various proposals
As part of the legislative process, the European Commission published a so-called ‘Impact Assessment‘ at the end of 2020. The Impact Assessment sets out the labeling rules that need adjustment, and the different options for amendment as proposed by the European Commission. The Impact Assessment for tightened regulations on alcoholic beverages labeling was published later, in the summer of 2021. Below we discuss which changes to the FIC Regulation are proposed in the Impact Assessments and why, and touch upon the options presented for each topic. Later this year, the European Commission will follow up with a proposal for an amended regulation.
FOP labeling
Consumers do not always understand the nutritional information on packaging, which makes it difficult to choose healthy foods. To help consumers making better decisions, there are all kinds of voluntary initiatives for clear front-of-pack (FOP) nutrition labeling. Examples include the much discussed Nutri-Score, but also other initiatives such as the traffic light system (UK), the keyhole (Scandinavia) and the battery (Italy). However, these diverse FOP logos do not necessarily help European consumers as they do not provide equal access to information. The European Commission is concerned that this could lead to fragmentation of the single market, costs for food companies operating in several member states, and confusion among consumers. Harmonizing FOP logos and making them mandatory or not, are options on the table.
Another proposed change concerns nutrition and health claims on unhealthy products. For example, consider the claim “source of fiber” for biscuits and “rich in vitamin C” for soft drinks. Such claims obscure the unhealthy profiles of these products, which leads to ‘health washing’ (term as introduced by the Dutch consumer association, see here). Nutrient profiles, i.e. thresholds for fats, sugars and/or salt above which the use of nutrition or health claims is restricted, could provide a solution to this. Such thresholds could help preventing consumer deception and create a level playing field for food companies. According to the Claims Regulation, nutrient profiles should already have been established as early as 2009, but this never materialized due to intense debate on the matter. The revision of the FIC Regulation revitalized this discussion. Based on stakeholder feedback on the Impact Assessment, the time seems ripe for change now.
Origin labeling
There is a growing demand from consumers to know the origin of food products. This allows consumers to make more sustainable choices, such as by choosing local products. Origin labeling is already mandatory for certain types of meat, among others. In the absence of harmonized rules regarding other food categories, a number of member states developed national rules for this purpose. Rules differentiating from member state to member state however lead to unequal access to information within the EU and fragments the internal market. The European Commission is therefore investigating whether and, if so, how, European rules on origin labeling could be further extended. This could mean an expansion of the product groups for which origin indication is required. Current discussions also include the production phase to which the indication should refer and the area size referred to (EU / non-EU, or e.g. country or regional level).
Date marking
We currently have two types of date marking. Whereas ‘use by’ refers to the expiry date for food safety reasons, ‘best before’ refers to the date by which the food retains its optimum quality. After this date, color variations may occur, for example, but the product is still safe to eat. The Impact Assessment states that less than one in two consumers understand the meaning of the two date markings. As a result, a lot of food ends up in the waste bin unnecessarily. By estimate 10% of the 88 million tons of food that is wasted annually is linked to date marking. In the context of improving sustainability by reducing food waste, the Impact Assessment presents options to educate consumers about date marking. It also suggests the possibility of removing the ‘best before’ quality date where this has little or no added value.
 Labeling of alcoholic beverages
Labeling of alcoholic beverages
Health damage from alcohol is a serious public health issue in the EU. To reduce alcohol consumption, it is important to inform consumers about what is actually in alcoholic beverages, both in terms of ingredients and nutrients. Currently, this is not mandatory for alcoholic beverages with an alcohol volume above 1.2%. Having said that, there are various initiatives for better information provision on alcohol, such as self-regulation within the beer and spirits sectors. To create a level playing field for operators, the European Commission communicated among others a proposal to remove the aforementioned labeling exception for all alcoholic beverages. Some of the then required information could be communicated off-label via a QR code.
Follow-up steps
Interested parties were able to share feedback with the European Commission after the publication of the two Impact Assessments. To further prepare the legislative proposal, a public consultation took place from 13 December 2021 to 7 March 2022. This process was designed to gather further views, experiences and suggestions on food labeling from stakeholders. As many as 3224 citizens, companies, interest groups, public authorities and other parties filled out the questionnaire made available for this purpose. A large proportion of respondents expressed their support for a harmonized FOP logo, as well as for improved terminology or a visual presentation of date marking. The European Commission’s legislative proposal is expected later this year.
This blogpost has also been published in Dutch at VMT.nl. The author thanks Marie-Claire Evers for her translation of this blogpost into English.
The era of nudging: EU’s proposals for mandatory front-of-pack labeling
Posted: June 13, 2022 | Author: Jasmin Buijs | Filed under: Advertising, Authors, Food, Information, Nutrition claims | Comments Off on The era of nudging: EU’s proposals for mandatory front-of-pack labelingAs part of the EU Farm to Fork Strategy for a sustainable food system under the Green Deal, the European Commission agrees that nudging is necessary to guide the consumer to healthier and more sustainable food choices. This has translated into two impact assessments for mandatory front-of-pack (FOP) labeling. Various EU Member States and individual food business operators are however not waiting for harmonized EU FOP labeling and adopted the nutri-score (guiding health choices) and/or eco-score (guiding sustainability choices). This blogpost shows how these initiatives fit into the current and upcoming legal framework.
Nutri-score
 Nutri-score is a five-color nutrition label demonstrating the overall nutritional value of a food product front-of-pack. It allows consumers to compare various foods in a simple and fast way. The label is based on a scale of 5 color and letters, from a dark green “A” for the most healthy choice to a red “E” for the least healthy choice. Simply put, the algorithm behind nutri-score allocates positive points for favorable dietary components (fruits, vegetables, pulses nuts, fibers and proteins) and negative points for energy and unfavorable dietary components (saturated fatty acids, sugars and sodium). The total positive points are subsequently subtracted from the total negative points. The lower the score, the better the letter/color grade.
Nutri-score is a five-color nutrition label demonstrating the overall nutritional value of a food product front-of-pack. It allows consumers to compare various foods in a simple and fast way. The label is based on a scale of 5 color and letters, from a dark green “A” for the most healthy choice to a red “E” for the least healthy choice. Simply put, the algorithm behind nutri-score allocates positive points for favorable dietary components (fruits, vegetables, pulses nuts, fibers and proteins) and negative points for energy and unfavorable dietary components (saturated fatty acids, sugars and sodium). The total positive points are subsequently subtracted from the total negative points. The lower the score, the better the letter/color grade.
Fighting nutrition-related non-communicable diseases
The aim of nutri-score is to nudge the consumer into healthier food choices, and to stimulate the food industry to reformulate their recipes. This way, nutri-score should contribute substantially to a reduced burden of nutrition-related non-communicable diseases such as diabetes, cardiovascular diseases and some types of cancer.
Legal basis
From a legal perspective, nutri-score qualifies as voluntary food labeling in accordance with article 36 FIC Regulation. Food business operators that opt for using nutri-score are however obliged to use it for all foods they place on the market to avoid cherry picking. Moreover, a green “A” or “B” score additionally qualifies as a nutrition claim under the Claims Regulation. Since the claim is not listed in the annex to the Regulation, Member States adopting the nutri-score are subject to the notification procedure of article 23 of the Claims Regulation.
Algorithm changes
Countries that implemented nutri-score (France, Belgium, Luxembourg, Germany and Switzerland) or are willing to use it (Netherlands and Spain) join forces to ensure that nutri-score is in line with the national dietary guidelines. To coordinate such, the abovementioned countries established a Steering Committee and Scientific Committee in February 2021. The Steering Committee is composed of two representatives from national authorities in charge of the nutri-score implementation in each country; the Scientific Committee includes one or two independent experts nominated by each country involved. On March 7 last, the Scientific Committee published its interim report in which it proposes a methodology for modification of the nutri-score algorithm to handle problematic food categories (fats and oils, fish and seafood, whole grain products, salt, sugar, beverages, and dairy products). The Scientific Committee aims at providing a fully revised version of the nutri-score algorithm before the summer. The Steering Committee will have the final say in the recommendations proposed by the Scientific Committee and, where relevant, will elaborate a support document for food business operators to facilitate the appropriation of algorithm changes by the end of the year.
Developments at EU level
In the meantime, the European Commission held a public consultation to introduce standardized mandatory FOP nutrition labeling as part of the revision of the FIC Regulation within the EU’s Farm to Fork strategy. In its impact assessment, it listed five options:
- Baseline (“business as usual”) – it remains possible to voluntarily use a public or private, non-harmonized, FOP label.
- Nutrient- specific labels (numerical) – a harmonized FOP label such as the Italian Nutrinform Battery, providing numerical information on the content of macro nutrients and the energy value of a food, as well as the percentage of the daily refence intake that it makes up for.
- Nutrient-specific labels (color-coded) – a harmonized FOP label such as the UK Multiple Traffic Lights, which is similar to the numerical label but in addition uses colors to classify the content of nutrients as green, amber or red.
- Summary labels (endorsement logos) – a harmonized FOP label such as the Keyhole used in Sweden, which can be applied only to foods that comply with certain beneficial nutritional criteria.
- Summary labels (graded indicators) – a harmonized FOP label such as nutri-score, providing an appreciation of a product’s overall nutritional value through a graded indicator.
The harmonized FOP nutrition label as listed under 2 – 5 above could be either voluntary or mandatory, which is still subject to debate. The impact assessment also mentions the possibility of having a policy mix rather than using one preferred option. Next to the outcome of the public consultation, the European Commission will take into account the comprehensive review on FOP nutrition labeling schemes by the Joint Research Centre (2020) on EFSA’s recent scientific advise on nutrient profiling. A legislative proposal is expected in Q4 2022.
Eco-score
 Eco-score can be seen as the equivalent of nutri-score in the field of sustainability. Just like nutri-score, eco-score is a French initiative. It shows the consumer the environmental impact of a food, using the same presentation as nutri-score in terms of colors and letters. The food’s environmental impact is measured in two steps. First, the environmental footprint is calculated using the Product Environmental Footprint (PEF) method, which is based on a Life Cycle Assessment (LCA). The PEF method takes into account 16 different impact categories, such as ozone depletion, land use and climate change. This eventually translates into a score between 0 and 100. Thereafter, bonus points can be added up or minus points can be deduced from this score. These extra points (positive or negative) are based on 4 additional criteria: (1) food production methods as measured through attributed third-party sustainability credentials such as organic certification, fairtrade or MSC, (2) recyclability of the packaging, (3) the provenance of ingredients, and (4) the stay-away from biodiversity-related issues such as overfishing and deforestation. The LCA takes place at product category level; the allocated bonus and minus points are related to the individual product.
Eco-score can be seen as the equivalent of nutri-score in the field of sustainability. Just like nutri-score, eco-score is a French initiative. It shows the consumer the environmental impact of a food, using the same presentation as nutri-score in terms of colors and letters. The food’s environmental impact is measured in two steps. First, the environmental footprint is calculated using the Product Environmental Footprint (PEF) method, which is based on a Life Cycle Assessment (LCA). The PEF method takes into account 16 different impact categories, such as ozone depletion, land use and climate change. This eventually translates into a score between 0 and 100. Thereafter, bonus points can be added up or minus points can be deduced from this score. These extra points (positive or negative) are based on 4 additional criteria: (1) food production methods as measured through attributed third-party sustainability credentials such as organic certification, fairtrade or MSC, (2) recyclability of the packaging, (3) the provenance of ingredients, and (4) the stay-away from biodiversity-related issues such as overfishing and deforestation. The LCA takes place at product category level; the allocated bonus and minus points are related to the individual product.
Legal basis: currently no specific rules
There is not yet a legal framework specifically dedicated to environmental claims, let alone a legal definition thereof. Environmental claims are currently enforced based on general rules, guidelines and self-regulation within the legal framework of unfair commercial practices and misleading advertisements, as discussed in our earlier blogpost. Interestingly, these different sources produce different definitions of environmental claims. The definition thereof in the Dutch Code for Environmental Advertising is for example very broad and includes the eco-score as a claim related to the environmental factors connected with the product. It is however questionable whether an eco-score “C”, “D” or “E” would fall under the definition of a ‘green claim’ under the EC Guidance on the Unfair Commercial Practices Directive. This latter guidance namely refers to a positive environmental impact (which products with lower scores have not) or a lower damaging impact on the environment than competing products. Since the eco-score algorithm is largely based on product categories rather than individual products, it is not necessarily suitable for comparisons between competing products such as for example different fruit juices. Assuming that eco-score does qualify as an environmental claim, the following question is whether it is in conformity with the applicable rules. These rules are however not black and white and leave room for interpretation, especially since the number of enforcement cases is still rather low.
Future situation: EU harmonization of eco-score?
The ambiguity illustrated above may be over with in the near future, since the European Commission is working towards the harmonized use of a sustainability label under its Farm to Fork Strategy and the transition towards a sustainable food system. In its impact assessment, it lists the following options:
- Baseline (“business as usual”) – No specific new actions, though existing initiatives on environmental claims will be continued, such as the upcoming legislation on the substantiating of environmental footprint claims by use of the PEF or OEF (organizational environmental footprint) method.
- Voluntary approaches – No legislative initiatives but guidance and private initiatives such as codes of conducts.
- Reinforcing existing legislation – Development of sustainability labeling provisions related to more than one sustainability component (such as environmental and social sustainability) through existing sector-specific legislation (for example fisheries marketing standards).
- Voluntary EU sustainability label – Development of a voluntary harmonized sustainability label, either applicable to all foods or to foods that meet a certain sustainability standard only.
- Mandatory EU sustainability label – Development of a mandatory harmonized sustainability label, either for all foods placed on the EU market or mandatory for EU produced foods and voluntary for imported foods.
A legislative proposal is expected in Q4 2023. Until July 21 of this year, it is possible to contribute through the public consultation.
Meanwhile in the Member States….
 Member States seem not to be waiting for the legislative proposals of the European Commission. Instead, various Member States launched national initiatives on FOP sustainability labels and join forces to ensure that such labels will be implemented in a similar way throughout the EU. Taking the Netherlands as an example, the Ministry of Agriculture, Nature and Food Quality aims to implement a voluntary sustainability label for foods in the Netherlands by 2025. This goal forms part of the National Climate Agreement. Together with the food sector and other stakeholders, the Ministry is currently investigating how such a label for the Dutch market could look like. LCA’s based on the PEF-method are taken as a starting point for further development.
Member States seem not to be waiting for the legislative proposals of the European Commission. Instead, various Member States launched national initiatives on FOP sustainability labels and join forces to ensure that such labels will be implemented in a similar way throughout the EU. Taking the Netherlands as an example, the Ministry of Agriculture, Nature and Food Quality aims to implement a voluntary sustainability label for foods in the Netherlands by 2025. This goal forms part of the National Climate Agreement. Together with the food sector and other stakeholders, the Ministry is currently investigating how such a label for the Dutch market could look like. LCA’s based on the PEF-method are taken as a starting point for further development.
Conclusion
FOP labeling is a topic of conversation. Various initiatives on both national and European level are taking place simultaneously, in the hope that they will once come together as an EU harmonized label. We see different food businesses reacting to this situation differently. Where some opt for awaiting formal decisions at national level and instructions by the government, others are pioneering and experimenting with FOP labeling within the currently existing legal framework. Examples include the nutri-score pilot by Iglo and the full-fledged use of eco-score by Colruyt Group in Belgium. What about you? Are you a game changer or a laggerd?
Together with Lisa Gray from Iglo and Veerle Poppe from Colruyt Group, Jasmin Buijs presented this topic at the VMT Food Law Event on June 7 last.
Making an environmental claim on a product? Stay away from these 7 sins of greenwashing
Posted: November 15, 2021 | Author: Jasmin Buijs | Filed under: Authors, Food, Information | Comments Off on Making an environmental claim on a product? Stay away from these 7 sins of greenwashing Food packaging materials (and other products) are more and more often advertised as environmentally friendly, compostable, recyclable, or otherwise sustainable. In general, this is a positive development: it helps consumers to make (more) sustainable choices. But there is also a danger lurking, namely greenwashing. Greenwashing occurs when a product (including packaging) is being advertised as more sustainable than it actually is. In such cases, the consumer is not helped by the information conveyed and in fact the opposite is true: the consumer is misled. This blogpost explains what environmental claims are, the applicable legal framework, and, most importantly, how to prevent greenwashing.
Food packaging materials (and other products) are more and more often advertised as environmentally friendly, compostable, recyclable, or otherwise sustainable. In general, this is a positive development: it helps consumers to make (more) sustainable choices. But there is also a danger lurking, namely greenwashing. Greenwashing occurs when a product (including packaging) is being advertised as more sustainable than it actually is. In such cases, the consumer is not helped by the information conveyed and in fact the opposite is true: the consumer is misled. This blogpost explains what environmental claims are, the applicable legal framework, and, most importantly, how to prevent greenwashing.
Definition of environmental claims
There is no legal definition of environmental claims. However, the term is described in the European Commission guidance on unfair commercial practices legislation and by Dutch self-regulation. In short, environmental claims refer to the practice of suggesting in commercial communications that a product or service has a positive or no impact on the environment or causes less damage to the environment than other products or services (for example by text, images or the use of color). The Dutch Code for Environmental Advertising clarifies that environmental claims may concern the entire life cycle of a product, from production to waste processing.
Environmental claims, also known as ‘green claims’, are not the same as sustainability claims. Both the Dutch Advertising Code Committee (in Dutch: Reclame Code Commissie, or ‘RCC’) and the Netherlands Authority for Consumers and Markets (in Dutch: Autoriteit Consument & Markt, or ‘ACM’) specified that ‘sustainability claim’ is an umbrella term including claims referring to environmental aspects, animal welfare and/or labor conditions.
Legal framework
Since there is no legal definition of environmental claims, it comes as no surprise that there is no specific legal framework either. At least not yet, because the regulation of environmental claims is a topic of debate under the European Green Deal. Having said that, at present companies making environmental claims are free to decide how to substantiate such claim. As a result of this, environmental claims are not comparable, more difficult to verify and not always reliable.
The fact that there is no specific legal framework does however not mean that no rules apply to environmental claims. Such claims must namely comply with general rules, guidelines and self-regulation on misleading advertisements. This includes first and foremost the Dutch Unfair Commercial Practices Act (in Dutch: Wet oneerlijke handelspraktijken), which prohibits misleading advertisements and which is supervised by the ACM. What the Dutch Unfair Commercial Practices Act means for environmental claims is explained in the above-mentioned guidelines of the European Commission. Furthermore, the ACM launched the Guidelines Sustainability Claims early this year, which document provides general tools to make clear, correct and relevant sustainability claims (and so environmental claims). With regard to food packaging materials in particular, the EU Framework Regulation on Food Contact Materials states that the labeling, advertising and presentation of a material or article must not mislead the consumer. Last but not least, there are several ISO standards that help meet the legal requirements for environmental claims. For example, ISO 14021:2016 contains requirements for so-called self-declared environmental claims, which are made without independent third-party certification. Based on this ISO standard, the European Commission developed a guideline to help formulating environmental claims and evaluating existing environmental claims.
The 7 sins of greenwashing
Environmental claims, unlike for example health claims for foods, are not part of a closed system with an exhaustive list of claims that can be made. On the one hand, this means that advertisers have more room to make such claims. At the same time, it means that there is more ambiguity about what is and is not allowed. The key question “What may and may not be claimed?” requires an individual assessment. What is clear, however, is that the following seven sins of greenwashing are absolute no-gos.
- Hidden trade-offs
Describing the product as ‘sustainable’ or ‘environmentally friendly’ on the basis of only a limited number of properties without considering the environmental impact of the total life cycle of the product. For example, if a new production process saves water while it increases the total footprint of the product in question, then a claim on the water saving is not justified.
- No evidence
Making environmental claims without providing easily accessible evidence that supports the claim. If a package is for instance being advertised as ‘CO2 neutral’, inform the consumer where the outcome of the underlying life cycle assessment (LCA) and/or other relevant test reports are explained in a way that is understandable to the consumer (e.g. on your company’s website).
Using terms that are too broad and/or too general to understand the correct meaning thereof. The more general a claim is, the higher the burden of proof is to substantiate the claim. Make environmental claims therefore as specific as possible.
- Use of false labels
Using designations and symbols that falsely give the impression that the claim has been externally verified. It is therefore preferred to make use of an existing quality mark rather than inventing your own designation or symbol. This also helps the consumer not to get lost in the jungle of quality marks.
- Irrelevant claims
Claiming something about the product that is technically true, but which is not important when looking at distinctions in terms of environmental performance. For example, glass is factually speaking BPA-free, but such a claim would be misleading since this substance never occurs in glass.
- Smaller evil
Claiming to be more sustainable than other products within the same category, while the sustainability of these types of products in general is in question. Consider, for example, an environmental claim on cigarette packaging. This is not done.
- Untruths
Claiming something about the product while it is not (entirely) true. Is the packaging for example recyclable or compostable? Substantiate this claim with a validated test before communicating this to the consumer.
Companies wishing to make environmental claims on food packaging materials (or other products or services) are advised to take into account the following tips. First, put yourself in the shoes of the consumer. Can there be confusion about the environmental benefit being claimed? If so, adjust the claim accordingly. Secondly, be honest. Make sure the environmental claim does not give the impression that the packaging is more sustainable than it actually is. Finally, support your claim with evidence. This could be done by having the packaging material and its environmental impact independently assessed. The results of such assessment should subsequently be expressed on the packaging in a way that the consumer can understand, or, if there is no room for this, on a website. In the latter case, don’t forget to make a reference to that website on the packaging!
A Dutch version of this blogpost has been published at VMT.
Authors: Jasmin Buijs, attorney-at-law at Axon Lawyers & Agnieszka van Batavia, Packaging Sustainability and Regulatory Advisor at The LCA Centre
Personalized nutrition as a medical device
Posted: July 8, 2021 | Author: Jasmin Buijs | Filed under: Authors, Disclosure of information, Food, Health claims, Information, Nutrition claims | Comments Off on Personalized nutrition as a medical device From May 26 this year, the personalized nutrition space has been enriched with a new piece of legislation: the Regulation (EU) 2017/745 on medical devices (hereinafter: “MDR”). The MDR is, generally speaking, applicable to all personalized nutrition (software) products, including apps and algorithms, with a medical intended purpose. Since most personal nutrition services that are currently on the market are lifestyle related, they will not be affected by the MDR. This will however be different for services that offer dietary advice for the prevention, alleviation or treatment of a disease. This blogpost aims to provide an introduction to the new rules on personalized nutrition as a medical device.
From May 26 this year, the personalized nutrition space has been enriched with a new piece of legislation: the Regulation (EU) 2017/745 on medical devices (hereinafter: “MDR”). The MDR is, generally speaking, applicable to all personalized nutrition (software) products, including apps and algorithms, with a medical intended purpose. Since most personal nutrition services that are currently on the market are lifestyle related, they will not be affected by the MDR. This will however be different for services that offer dietary advice for the prevention, alleviation or treatment of a disease. This blogpost aims to provide an introduction to the new rules on personalized nutrition as a medical device.
What is the MDR?
The MDR comprises of a set of rules that governs the full medical devices supply chain from manufacturer to end user in order to ensure the safety and performance of medical devices. It applies to any apparatus, application, software or other article intended by the manufacturer to be used for a medical purpose. This includes, like under the old medical devices legislation, the diagnosis, prevention, monitoring, treatment or alleviation of a disease. Since the application of the MDR, also devices designed for the prediction or prognosis of a disease qualify as a medical device. Genetic testing kits for medical purposes and other medical devices to be used in vitro for the examination of specimens such as blood are covered by the MDR’s sister, Regulation (EU) 2017/746 on in-vitro diagnostic medical devices (hereinafter: “IVDR”). The IVDR applies from May 26 next year.
When does the MDR apply to personalized nutrition software?
The essential question for the MDR’s applicability is thus whether the article or software concerned has a medical purpose or is merely lifestyle or well-being related. For example, an app that provides dietary advice based on the users’ preferences and potential health data is a lifestyle product. But if the same app claims to help address obesity or treat hypertension, then it can be linked to a disease and transforms into a medical device. While the intention of the manufacturer of the device is leading for the application of the MDR, it should be noted that only a statement that a product is not a medical device, or is meant for lifestyle purposes only, does not constitute a reason to escape from this regulation. The intended purpose is inferred from every document and statement that expresses the intended purpose, including advertising and marketing material. Disclaiming a medical intended purpose in the instructions for use but claiming such purpose in marketing materials will still result in a finding of a medical intended purpose. The MDR contains an explicit prohibition on statements that may mislead the end user about the intended purpose or ascribes functions or properties to the device that it does not have, which would be caught in its scope.
Another example of a medical device is the wearable being developed by the Australian start-up Nutromics, which assesses dietary biomarkers to provide dietary advice to minimize users’ risk for lifestyle-related chronic diseases. This device is designed to predict the user’s likelihood of developing a particular disease and to subsequently provide advice to prevent such disease, and will therefore be subject to the MDR when placed on the Union market. On the other hand and as clarified earlier by the European Commission in its borderline manual for medical devices, a home-kit that enables the user to ascertain their blood group in order to determine whether a specific diet should be followed falls outside the medical sphere (as defined in the (old) medical devices legislation). The sidenote should however be made that this decision was not related to following a specific diet for medical purposes.
It is also relevant to state that software may as well qualify partly as a medical device and partly as a non-medical device. Think for example of an app that provides the user with personalized nutritional advice as part of the treatment or alleviation of diabetes, and additionally facilitates online contact with fellow patients. While the software module(s) that provide information on the treatment or alleviation of the disease will be subject to the MDR, the software module that is responsible for the contact amongst patients has no medical purpose and will therefore not be subject to the MDR. The manufacturer of the device is responsible for identifying the boundaries and the interfaces of the medical device and non-medical device modules contained in the software. The Medical Device Coordination Group has pointed out in its guidance on qualification and classification of software (October 2019) that if the medical device modules are intended for use in combination with other modules of the software, the whole combination must be safe and must not impair the specified performances of the modules which are subject to the MDR. This means that also the non-medical devices module(s) may have to be covered in the technical documentation that supports the device’s compliance with the MDR and as further introduced below.
Way to market of personal nutrition software
All medical devices placed on the Union market must in principle have a CE (conformité européenne) marking as proof of compliance with the law. To apply the CE-mark, the manufacturer has to prepare technical documentation and carry out a conformity assessment procedure, including clinical evaluation. The specific procedure that must be followed depends on the risk class of the device. The MDR distinguishes the risk classes I, IIa, IIb and III (from low to high). The higher the risk class of the device, the more stringent requirements for clinical evaluation apply to demonstrate safety, performance and clinical benefits. It could ironically be stated that MDR stands for More Data Required: where an analysis of available clinical data from literature may have been sufficient in the past, the MDR more often requires own clinical investigations to support the device’s safety and performance.
Under the MDR, software as medical device is classified as class IIa when it is intended to provide information which is used to take decisions with diagnosis or therapeutic purposes, or to monitor physiological processes. Translated to personal nutrition services, this would for instance include apps that track burned calories (monitoring of physiological processes) and that provide dietary advice as part of a treatment (therapeutic purposes). Risk increasing factors will however lead to a higher risk class than class IIa. While an app with which food products’ barcodes can be scanned to detect certain ingredients for allergen control purposes would normally be a class IIa device, it will be a class III device when food intake decisions based on the app may cause life-threatening allergic reactions when based on a false negative (safe to eat while it in fact is not safe).
All other software which is not specifically addressed but still has a medical intended purpose in scope of the definition of a medical device, is classed as class I. An example would be an app that provides nutritional support with a view to conception. The risk classification rules for software are new under the MDR, which means that existing medical devices software may be scaled in a higher risk class since 26 May 2021. This is relevant not only with regard to the conformity assessment procedure that must be followed, but also to the question who can perform the required assessment of legal compliance. Manufacturers of devices in a higher class than class I must always engage a so-called notified body in this regard, while class I devices can in principle be self-certified by the manufacturer. An exception exists however for class I devices that have a measurement function, for example by using the lidar of the user’s phone to measure the body as input for the functionality of the app, which also require the engagement of a notified body.
Take away for food businesses
When personal nutrition enters into the medical sphere, the MDR will come into play. This will impact first and foremost the manufacturers, importers and distributors of actual medical devices, including apps and algorithms. Having said that, food businesses that wish to market their food products through medical devices apps are advised to be aware of the implications thereof. This will help them to find a reliable partner and to ensure their interests are contractually protected.
A more elaborate version of this blogpost has been published at Qina in cooperation with Mariette Abrahams.
Towards 100 % recyclable or re-usable plastic food packaging in 10 years
Posted: May 4, 2020 | Author: Jasmin Buijs | Filed under: Authors, Food, Information | Comments Off on Towards 100 % recyclable or re-usable plastic food packaging in 10 yearsWhat would food be without packaging? And what does packaging mostly consist of? Of course: plastic! To date, only few other materials have proved as reliable for food packaging as plastic. At the same time, we know that plastic has many disadvantages for the environment. Read this blogpost to be informed on the most important initiatives of the food sector and on the legal tools of the European Commission in this field.
The visionary n umber of 2030
umber of 2030
By 2030, all plastic packaging in the EU will be recyclable or re-usable. In other words, all plastic packaging will then be collected and transformed into new packaging materials, or can be used as such more once. This goal follows, amongst others, from the European Strategy for Plastics. It is a worthy endeavor, and for some it is not even ambiguous enough. The signatories of the Plastics Pact aim to achieve this result as early as 2025. Nestlé has also announced its ambition to make 100% of its packaging recyclable or re-usable by 2025. Since this year, Lipton bottles sold in the Netherlands are already made from 100% recycled plastic. The so-called European Single-use plastics Directive (Directive 2019/904) will soon be introduced to further promote recycling of plastics as one of the measures against disposable plastics. In the Netherlands, this Directive will be implemented by means of a General Administrative Order (in Dutch: Algemene Maatregel van Bestuur, or “AMvB”) before the Directive’s date of application on 3 July 2021. In this regard, an internet consultation will be launched this month to invite citizens, companies and institutions to make suggestions to improve the quality and feasibility of the current proposal.
Recycled materials and articles
The encouragement to use recycled materials and articles in the EU for environmental reasons was already expressed in recital 24 of the European framework legislation on food contact materials (Regulation 1935/2004). However, food safety and consumer protection should not be neglected. Therefore, food packaging materials, including recycled plastics, should not endanger human health and not adversely affect the composition of foods or their sensory properties in an unacceptable way. The above-mentioned framework legislation leaves room for detailed rules on specific materials, and priority has been given to rules on recycled plastics. Their use has been increasing for a long time. Indeed, today’s sustainability initiatives cannot be imagined without the recycling of plastic food packaging. Moreover, diverging national legislation on this topic as a consequence of no harmonization at EU level would be highly undesirable.
Specific legislation
Specific rules on recycled plastic materials and articles intended to come into contact with foods can be found in Regulation 282/2008. Such rules are important because plastic packaging waste can be contaminated by residues from previous use, but also by contaminants from misuse and from non-authorized substances. In order to ensure that possible contamination is removed and that recycled plastics therefore do not adversely affect human health and foodstuffs with which they come into contact, an appropriate sorting and recycling process is essential. It should be noted that Regulation 282/2008 refers to mechanical recycling, which does not involve any significant change in the chemical structure of the plastic material. This process is less suitable for composites and multilayered plastics, which means that not all plastics are eligible for recycling under Regulation 282/2008.
Authorization procedure plastic recycling processes in a nutshell
Before a recycling process can be applied to plastics to create food packaging, the process must be approved by the European Commission. Such authorizations are process specific. This means that authorizations cover a specific recycling process used in a specific company by applying specific technologies and process parameters. Authorization holders are of course free to license out the authorized recycling process to other companies. Applications for the approval of recycling processes shall be submitted to the competent authority at Member State level. The European Commission published a list of contact points for competent authorities. In the Netherlands, applications can be submitted to the Ministry of Health, Welfare and Sport (in Dutch: Ministerie van Volksgezondheid, Welzijn en Sport, or “VWS”) and the National Institute for Public Health and the Environment (in Dutch, Rijksinstituut voor Volksgezondheid en Milieu, or “RIVM”).
As part of the application, a technical dossier is to be provided. This dossier will subsequently be subject to a safety assessment by EFSA, followed by a scientific opinion. In fact, here we very much see the parallel with the safety evaluations EFSA performs regarding for instance Novel Foods and additives. Topics to be covered to evaluate the safety of a particular recycling process include input characterization, description of the recycling process, determination of the decontamination efficiency of the recycling process, output characterization, and intended application in contact with food. For example, the dossier must demonstrate that and how it is ensured that the input material does not contain any chemical substances that could survive the recycling process and migrate to our foods in irresponsible quantities. In order to evaluate potential migration, detailed information on the type of food(s) intended to come into contact with the final product shall be provided. This will also include information on time, temperature and contact surface. Another important aspect concerns single or repeated use of the final product. All of this data serves to exclude possible health hazards and unacceptable influences on foods due to migration of substances. Further details can be found in the guidelines published by EFSA.
After receipt of a valid dossier, EFSA has, in principle, six months to evaluate the dossier. Thereafter, it is up to the European Commission to take an authorization decision. In doing so, it will take into account EFSA’s scientific advice. The final authorization may be subject to conditions and/or restrictions, such as with regard to the plastic input, the recycling process and/or the field of application of the recycled plastic. Authorized recycling processes are listed in a Community register established by the European Commission.
100% R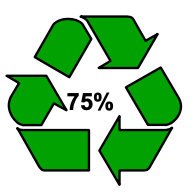 ecyclable
ecyclable
Many companies like to inform consumers that the food packaging they use is made from recycled plastics. However, it is essential that consumers are not misled about, for example, the actual content of recycled plastics used. Companies are therefore advised to follow ISO 14021 on environmental labels and declarations, or equivalent rules. In addition, the Dutch Advertising Code Committee (in Dutch: Reclame Code Commissie, or “RCC”) also explains what is possible and what is not, such as regarding packaging made of recycled plastics being environmentally friendly or friendlier. Environmental claims, i.e. advertisements in which implicit or explicit reference is made to environme ntal aspects associated with the production, distribution, consumption or waste processing of goods or services, must be verifiable. For instance, communicating that an article is “good for nature” because of the material used is perceived as misleading when this statement cannot be checked. It is no problem to use environmental symbols, as long as there is no confusion about the symbol’s origin and meaning. Examples of this include the Mobius Loop accompanied by the percentage of recycled material used in or near the symbol, as well as Lipton’s variation thereof.
ntal aspects associated with the production, distribution, consumption or waste processing of goods or services, must be verifiable. For instance, communicating that an article is “good for nature” because of the material used is perceived as misleading when this statement cannot be checked. It is no problem to use environmental symbols, as long as there is no confusion about the symbol’s origin and meaning. Examples of this include the Mobius Loop accompanied by the percentage of recycled material used in or near the symbol, as well as Lipton’s variation thereof.
Conclusion
While food business operators shall normally not be involved in the recycling of packaging themselves, they are advised to closely follow developments in this regard. Packaging namely plays an essential role in protecting foodstuffs and in providing information about the food it contains. Food business operators will therefore benefit from being aware of sustainable packaging possibilities. Moreover, food business operators can strengthen their legal position by being familiar with the legal requirements of the parties they cooperate with as well as with their own responsibilities set out by law. An example of the latter concerns making verifiable and non-misleading environmental claims, which is not always as easily done as said.
Transparency vs. Confidentiality: EFSA’s new role as from March 2021
Posted: July 31, 2019 | Author: Jasmin Buijs | Filed under: Authors, Food, Information, novel food | Comments Off on Transparency vs. Confidentiality: EFSA’s new role as from March 2021 Last month, the European Council formally adopted the new Regulation on the transparency of the EU risk assessment in the food chain, which will be applicable as of 26 March 2021. As is in the name, the new provisions aim at increased transparency of EU risk assessment, which for a large deal means strengthening the reliability, objectivity and independence of the studies used by European Food Safety Authority (“EFSA”). As EFSA has been accused more than once of conflicts of interest, this is, in principle, a welcome development. For FBOs, the implications are however important – as will be demonstrated below.
Last month, the European Council formally adopted the new Regulation on the transparency of the EU risk assessment in the food chain, which will be applicable as of 26 March 2021. As is in the name, the new provisions aim at increased transparency of EU risk assessment, which for a large deal means strengthening the reliability, objectivity and independence of the studies used by European Food Safety Authority (“EFSA”). As EFSA has been accused more than once of conflicts of interest, this is, in principle, a welcome development. For FBOs, the implications are however important – as will be demonstrated below.
The upcoming changes follow from the Commission Communication on the European Citizens Initiative on glyphosate, in which initiative EU citizens called for more transparency in scientific assessments and decision-making, and build upon the findings of the fitness check of the General Food Law. The new provisions will mainly amend the General Food Law. For reasons of consistency, the new provisions also introduce changes to eight legislative acts dealing with specific sectors of the food chain, including the Novel Food Regulation, the Additives Regulation and the GMO Regulation. Based on the publicly available proposal for the meanwhile adopted provisions on the transparency of EU risk assessment, this blogpost provides an overview of what applicants will be facing in authorization procedures for innovative food products in terms of transparency and confidentiality.
Pre-submission phase: union register of commissioned studies and general advice
Before submitting an authorization application, for instance for a Novel Food like cultured meat or insects or for a new additive, applicants will have to notify EFSA of any study they commissioned to support a future authorization application. These studies will become part of the union register of commissioned studies as managed by EFSA. The submitted studies will be made public only when an actual application follows and insofar the studies do not contain confidential information, regarding which a request for confidential treatment has been granted. The idea behind the union register is that companies (potentially) applying for an authorization submit all related information, which subsequently allows EFSA to cross-check the information on the studies performed. This way, applicants can no longer hold back unfavorable studies. This notification obligation also applies to laboratories in the EU that carry out those studies, but obviously not to non-EU laboratories as they fall outside the scope of new provisions. According to the EC’s fact sheet of 13 June 2019, consequences of non-compliance with the notification obligation shall result in a negative temporary stop in the risk assessment.
At the same time, applicants have the right to request EFSA for advice on the relevant provisions and the required content of the application for authorization in the pre-submission phase. This procedure is a response to industry demand, especially of SMEs, for further support in the preparation of applications. The advice shall, however, be provided without the input of the Scientific Panels that are in charge of the actual scientific assessment and shall not cover the specific design of a study. Furthermore, EFSA’s advice shall be made public. Based on the publicly available proposal for the transparency provisions, it seems that this will be done before an actual authorization application is being submitted.
Submission phase: citizens’ access to studies vs. confidentiality
 The default setting under the new provisions is that all scientific data, studies and other information supporting applications for authorizations shall be made public by EFSA. While the new provisions aim at ensuring that stakeholders and the public have access to key safety related information being assessed by EFSA, duly justified confidentiality information shall not be made public. The new rules specify which types of information may be considered as such. As listed in the publicly available proposal, this includes production methods, the quantitative composition of the substance or product at stake, certain commercial relationships and other sensitive business information. It is the responsibility of the applicant to demonstrate that making public the information concerned would significantly harms the (commercial) interests concerned. Only in very limited and exceptional circumstances relating to foreseeable health effects and urgent needs to protect human health, animal health or the environment, confidential information shall be disclosed.
The default setting under the new provisions is that all scientific data, studies and other information supporting applications for authorizations shall be made public by EFSA. While the new provisions aim at ensuring that stakeholders and the public have access to key safety related information being assessed by EFSA, duly justified confidentiality information shall not be made public. The new rules specify which types of information may be considered as such. As listed in the publicly available proposal, this includes production methods, the quantitative composition of the substance or product at stake, certain commercial relationships and other sensitive business information. It is the responsibility of the applicant to demonstrate that making public the information concerned would significantly harms the (commercial) interests concerned. Only in very limited and exceptional circumstances relating to foreseeable health effects and urgent needs to protect human health, animal health or the environment, confidential information shall be disclosed.
Specific sectoral food legislation will also include a list of certain types of information that may be treated confidentially. For example, a proposed change to the GMO Regulation includes the specification that certain DNA sequence information and breeding patterns and strategies may be treated confidentially. Next to the confidentiality rules, existing intellectual property rights, data protection rights and data exclusivity provisions for proprietary data, remain applicable.
When an applicant submits a request for confidentiality, it shall 
provide a non-confidential version and a confidential version of the submitted information. If and when EFSA receives the submitted information, for example in case the Commission requires a scientific opinion as part of a novel food procedure, it shall publish the non-confidential version without delay and shall decide on the requested confidentiality within 10 days. Insofar the confidentiality request has not been accepted, EFSA shall subsequently publish the additional information after 2 weeks from the notification of its decision. Applicants that do not agree with EFSA’s decision may, under certain circumstances, challenge the decision before the Court of Justice of the European Union.
Once EFSA has published all the non-confidential information and before it carries out its scientific assessment, EFSA may consult third parties, including citizens, to identify whether other relevant information is available on the substance at stake. This new provision serves to mitigate the public concerns that EFSA’s assessment is primarily based on industry studies, more in particular on studies ordered by the applicant. The results of these consultations shall also be made public, as will be minority opinions to EFSA’s scientific output.
Renewals
In case of a request for the renewal of an authorization, the applicant of the renewal will have the obligation to inform EFSA on its planned studies under the new rules. EFSA shall subsequently consult stakeholders and the public on the planned studies and, taking into account the received comments, provide the applicant with advice on the content of the intended renewal application. The reason behind this pre-submission procedure is to make use of the existing experience and knowledge on the substance or product in question. The European Commission expects that the notification obligation will have a positive effect on the evidence base of EFSA, avoids the unnecessary repetition of studies, and will provide the potential applicant with useful advice. It should, however, be noted that also in this case EFSA’s advice will not stand in the way of the subsequent assessment of the renewal application by the Scientific Panels.
Additional controls on the conduct of studies
The new provisions also include measures to ensure the quality and objectivity of the studies used by EFSA for its risk assessment. First of all, the European Commission will have the right to perform controls, including audits, to verify the compliance of testing facilities with relevant standards. Competent authorities of the Member States will be involved in these controls. Through coordination with OECD GLP auditing programs, the control in non-EU countries will be facilitated as well. Secondly, the European Commission will have the right to request EFSA to commission verification studies. Such action shall only be taken in exceptional circumstances, such as in case of serious controversies or conflicting results, and will be financed by the EU.
Conclusion
The new provisions address public concerns regarding transparency, while on the other hand acknowledge that confidentiality is key to not cut down on incentives for innovation by food businesses. In other words: the new provisions seek to balance transparency and confidentiality. Food businesses are advised to substantively consider their commercial interests when submitting a dossier for authorization, since confidentiality will only be granted upon duly justified requests. Moreover, the union register for submitted studies forces food businesses to carefully plan their studies at an early stage since also studies with less favorable outcomes will in principle be made public and scrutinized on inconsistencies with later studies. At the same time, more guidance is expected from EFSA in the pre-submission phase, possibly speeding up the actual authorization procedure. Given the fact that the new provisions will apply from less than 20 months from now, food businesses may consider speeding up or delaying their application for authorization, depending on their needs.



