The Role of Razzies in EU Food Law
Posted: November 4, 2013 Filed under: Food, Information Leave a comment »One day before the distribution of the Academy Awards for excellence of cinematic achievements, the ceremony of Golden Raspberries or “Razzies” takes place. Razzies are awarded in recognition of the worst in film. Obviously, no producer, actor or actress is looking forward to receiving this “prize”. However, Razzy ceremonies continue to take place ever since 1980, so there seems to be no escape.
Razzies in Food Law
Since 2009, the Razzies have their peer in EU food law. Foodwatch, a European consumer protection organization active in Germany, France and the Netherlands, awards the so-called Golden Creampuff. This prize is granted for marketing strategies designed to “cleverly obscure the discrepancy between the alleged positive qualities of the products in question and their actual benefits.” For a number of years, this “prize” has been granted in Germany and the Netherlands under the names Goldener Windbeutel and Gouden Windei respectively.
Gouden Windei competition
On 3 October 2013, the Gouden Windei was awarded to the Dutch coffee company Douwe Egberts for a sweetener from its Natrena Stevia product line. Douwe Egbert’s product competed with four other products consisting of an energy drink, a cake, a salmon dinner product and a pear ice cream.The Natrena Stevia product line offers alternatives for sugar based on steviol glycosides that are extracted from the leaves of the Stevia plant. Douwe Egberts previously only marketed sweeteners based on Aspartame, but some consumers have a preference for the natural ingredient Stevia over the chemical compound Aspartame. You may recall that aspartame was regularly the object of public concern due to anecdotal reports of adverse effects. So far however, EFSA has concluded that it is safe to use this product as a food additive.
Natrena Stevia is the winner
Douwe Egberts’ winning product consisted of a small jar containing 70 grams of “Natrena Stevia crystal powder”. In total, 14.322 consumers had participated in the election of the Gouden Windei 2013, which election featured in total five products. 27 % of those consumers (3.866 persons) considered the Natrena Stevia sweetener to be the most misleading, as it only consists of 3 % Stevia. The other 97% of the product consists of Maltodoxtrine, which is a carbohydrate produced from potato or corn starch.
Applicable legal framework
The applicable legal framework, with which products such as Natrena Stevia have to comply, is in the first place constituted by the Food Information Regulation. This Regulation will replace the current labeling Directive 2000/13 on 13 December 2014. However, in order to ensure a smooth transition, many food operators tend to act in compliance with the Food Information Regulation as of today. According to article 22 of this Regulation, the indication of the quantity of an ingredient used in the manufacture or preparation of a food shall be required where the ingredient concerned is emphasised on the labelling in words, pictures or graphics. At a national level, similar provisions apply. The product Natrena Stevia features a list of ingredients indicating that this product contains 3 % Stevia. At this point, this products therefore seem to comply with the applicable legislation.
And what else?
In as far as food products do not comply with the applicable legal framework, consumer complaints can be justified and one could argue that organisations such as Foodwatch do a good job. However, it is all the more interesting to see that consumer complaints are also mobilised against products that in fact are in compliance. Reference is made to a case recently decided by the Dutch Advertising Code Authority that was previously discussed on this blog. Should it be concluded that the provisions laid down in the Food Information Regulation only provide a de minimis framework that does not pave the way for full consumer information? Or it is justified to expect from consumers of food products that they do some further research in addition to the information perceived at first glance?
Public perception
One can wonder if the yearly Razzies constitute an isolated momentum or if they have some social or economic impact indeed. Based on the changes applied in the information provided currently provided with the Natrena Stevia product, this latest Razzy seem to have had repercussions indeed. Douwe Egberts added an explanatory note on its website regarding the limited quantity of Stevia contained in its product Natrena Stevia crystal powder. In free English translation this reeds “Stevia crystal powder consist of the light filler maltodextrine (97%) and Stevia (steviol glycosides 3 %). Why so little Stevia? That is because Stevia is 300 times sweeter than sugar and in its pure form therefore is difficult to dose. The filler causes 1 small spoon of Natrena Stevia to be as sweet as a small spoon of sugar, but it contains far less calories (3kcal per tea spoon). Based on all of this, it can be concluded that public perception, in addition to all legal and other product requirements, also plays a role in EU Food Law. Hopefully for the benefit of our healthy appetite!
New Labelling Regulation: more COOL?
Posted: October 30, 2013 Filed under: Food, Information | Tags: country-of-origin, horsemeat, information, labelling Leave a comment »Rumour has it that the European Commission will decide not to go for full country-of-origin labelling (‘COOL’) on all meat products in the European Union. According to globalmeatnews.com the European Commission is planning to recommend only partial country-of-origin labelling for fresh meats. This is not in line with the request of MEPs and EU ministers who have explicitly asked to beef up the new Labelling Regulation with better meat origin labelling for both unprocessed and processed meats. Why does the Parliament pressure the Commission to impose origin-labels on processed meat?
The reason for the Parliament’s pressure: the horsemeat scandal
The horsemeat scandal has been making headline news over the last year. It was a scandal caused by fraudulent labelling. Horsemeat was present in beef products and the consumer was not informed about this. For a Dutch perspective on the horsemeat scandal, see this article (in Dutch).
To restore consumer confidence and to improve controls the Commission has launched an Action Plan. COOL is part of the Action Plan and the Parliament has been pushing the Commission to adopt mandatory origin labelling for both fresh meat and meat in processed food (meat as an ingredient). In April, and later in September 2013, MEP Glenis Willmott urged the Commission to put rules on country-of-origin in place on ‘country of origin’ labelling for meat in ready meals and in processed foods. This month Health Commissioner Tonio Borg received a letter from MEP Agnès Le Brun to further pressure the European Commission for better meat origin labelling. The MEPs main arguments are that the horsemeat scandal highlighted the need for honest food labelling and by using COOL producers would have a much better grip on their supply chain.
Could COOL ensure honest labelling and prevent fraud?
In March 2013 Commissioner Tonio Borg answered this question in an interview. According to Borg the horsemeat scandal should be seen for what it is: a fraud rather than a demonstration of a regulatory gap. COOL would not necessarily create another hurdle for fraudsters: “(…) one could be honest about the origin but fraudulent about the labelling on the ingredients.” If you want to see the video of the interview, click here. I agree with Borg. The horsemeat scandal was a case of intended mislabelling, COOL will not prevent it from happening again.
Country-of-origin: current EU legislation
The current Labelling Directive only requires the place of origin or provenance to be mentioned on the label where failure to give this information might mislead the consumer (article 3(8)). In the EU, the origin must always be labelled for olive oil, fish (unless it is canned or prepared), beef, fresh or frozen poultry of non-EU origin, wine, most fresh fruit and vegetables, honey and eggs. For all other foods, origin labelling is optional.
New Labelling Regulation
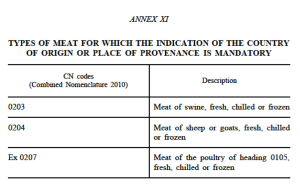
According to article 26 of the new Labelling Regulation, indication of the country of origin shall be mandatory where failure to indicate this might mislead the consumer and for meat listed in Annex XI (see below).
The following steps have to be taken by the European Commission from now:
Autumn 2013 – Adopt a Commission report on the possibility to extend mandatory origin labelling of all types of meat used as ingredient in foods and take any necessary follow up action.
December 2013 – Adopt implementing rules on the mandatory origin labelling of unprocessed meat of sheep, goat, pig and poultry, based on the new Labelling Regulation.
December 2013 – Adopt implementing rules to prevent misleading use of voluntary origin labelling in foods, based on the Regulation on Food information to consumers.
December 2014 – Adopt Commission reports, based on the new Labelling Regulation, on the possibility to extend mandatory origin labelling to:
- other unprocessed meats not already covered by mandatory origin labelling rules, such as horse, rabbit, game meat etc.;
- milk;
- milk as an ingredient in dairy products;
- single ingredient foods;
- unprocessed foods;
- ingredients that represent more than 50% of a food.
So far, no official report from the European Commission on the meat labelling subject has been released, but it is expected to be published soon. Anyway, you will see an update here as soon as the report is published.
More COOL?
The Commission has not given a clear position on COOL yet, but if the rumours are true, the Commission is set to propose COOL for fresh pork, poultry and lamb. Compared to the current legislation, this results in more COOL because the requirements are being rolled out from beef to other meats, which will have an impact on the meat industry. But not totally COOL as the Parliament wants to see it. At this point it looks like COOL for processed meat will be rejected, which probably will be substantiated through an impact assessment. COOL for processed meat is likely to turn out to be too costly for the industry, and the consumer might not want to pay the price for origin information. Of course this is still speculative. Stay tuned for the update!
How to advertise healthy berries without cherrypicking?
Posted: September 25, 2013 Filed under: Food, Information Leave a comment » How do you communicate the main characteristics of your product to the public? First of all by its name of course – fair enough. At second glance, the list of ingredients comes into play. Pretty simple, isn’it? Beware however that Regulation 1169/2011 on the provision of food information to consumers (“the Regulation”) provides detailed rules for these two items – and more. And that you need to act in compliance by 13 December 2014, both regarding your product labels and regarding the information provided on your company’s website. If you are of the opinion that you can use a little help from this perspective, this post may be useful to you.
How do you communicate the main characteristics of your product to the public? First of all by its name of course – fair enough. At second glance, the list of ingredients comes into play. Pretty simple, isn’it? Beware however that Regulation 1169/2011 on the provision of food information to consumers (“the Regulation”) provides detailed rules for these two items – and more. And that you need to act in compliance by 13 December 2014, both regarding your product labels and regarding the information provided on your company’s website. If you are of the opinion that you can use a little help from this perspective, this post may be useful to you.
Name of the food
If there is a name prescribed by law for a food product, this must be used, e.g. “herring”. In the absence of such legal name, the customary or a descriptive name shall be used. This is a description of the food, and if necessary of its use, which is sufficiently clear to enable consumers to know its true nature and distinguish it from other products with which it might be confused, e.g. “cookie with Brazil nuts”.
Name in Member State of production equals name in Member State of marketing
In general, the name of the food in the Member State of production will be the same as the name in the Member State where the food is marketed, unless such does not enable the consumer to know the true nature of the food. For example, the name “vegetable samosa” may need to be qualified by “a pastry parcel with spicy vegetable filling”.
Other particulars to be communicated
Furthermore, the name of the food shall include or be accompanied by the particulars as to the physical condition of the food or the specific treatment which it has undergone in all cases where omission of such information could mislead the consumer, e.g. “freeze-dried” or “quick-frozen”. Also, in the case of foods in which a component or ingredient that consumers expect to be normally used or naturally present has been substituted, the labeling shall bear a clear indication of the component/ingredient that has been used for the partial of whole substitution. This should be done in close proximity to the name of the product and in a font size that is not much smaller than the name of the product. Finally, meat and fishery products giving the impression to be made of a whole piece of meat or fish, but actually consist of different pieces combined with other ingredients, shall bear the indication “formed meat/fish”. Bon appetit!
List of ingredients and legibility requirements
The list of ingredient shall include all ingredients of the food, named by their specific name, in descending order of weight, as recorded at the time of their use in the manufacture of the food. Only ingredients constituting less than 2 % of the finished product may be listed in a different order after the other ingredients. Futhermore, ingredients which belong to certain categories, e.g. “mixtures of flour obtained from two or more cereal species”, may be designated by the name of that category rather than by the specific name. As far as information present on the product label is concerned, this shall be printed on the package or on the label in such a way as to ensure clear legibility. Therefore, the x-height of het characters used shall in principle be equal to or greater than 1,2 mm.
Both product label and website information should be complete
Even if your product labels comply with all of the above, you may still be subject to a complaint regarding misleading information if your website information is not complete. In a recent decision of the Dutch Advertising Code Committee, a complaint regarding the product “Healthy People’s blueberry and raspberry juice” was discussed. According to this complaint, both the packaging and the company website provided misleading information regarding this product. The claim regarding the packaging was aimed at the fact that it only displayed blueberries and raspberries, whereas in reality, the juice consisted of 10 % blueberry and 4 % raspberry. From the list of ingredients, it appeared that other ingredients were apple, white raisin and aronia. The claim regarding the website was directed against the fact that blueberry and raspberry were the only ingredients mentioned.
Characteristic taste of the product
As a defence, Healthy People argued regarding both the packaging and its website that it was a deliberate choice to insist on the characteristic taste of the product rather than on side-issues. Besides, the consumer was considered smart enough to understand that a juice consisting of only blueberries and raspberries would be too sour and too expensive (the average price of a liter blueberries being EUR 15). Healthy People so far had not received any consumer complaints and it therefore had not realised there was some issue here.
Average consumer
The Advertising Code Committee considered that the packaging was in line with applicable legislation. Allegedly, the average consumer would understand that the product does not only consist of blueberries and raspberries. Instead, such consumer understands that these ingredients are, amongst others, part of the product. Such could be deduced from the list of ingredients that the average consumer can be expected to consult regarding a high end fruit drink. Now that the website did not contain such list of ingredients, this was considered unclear. In the absence of full information a consumer could decide to buy this product, whereas he would not have done so otherwise. The limited information provided on the website was therefore considered misleading.
Conclusions
As follows from the decision discussed above, it is not sufficient that the labeling is in compliance with the applicable food information legislation, the same applies to the information provided at your company’s website. As of December 2014, many detailed food information rules will apply under the Regulation. However, despite the aim for harmonization at an EU level, there is still room for interpretation. This for instance applies to name of the product in connection with the wording “where omission of such information could mislead the consumer”. It does not come as a surprise that the interpretation of such wording may vary from country to country. Obtaining local advice for the launch of a new product therefore continues to be a sound plan. Also, do not overlook the annexes attached to the Regulation. They are 15 and full of strict requirements, for instance the one to state “formed meat” on your product. Although not so sexy, when applicable, you will not be able to get around it!
Food Information on Allergens: no escape!
Posted: September 17, 2013 Filed under: Food, Information | Tags: allergen, allergens, food, information, labeling Leave a comment » Your company wants to provide bullet proof information on the food products it offers for sale in view of the upcoming changes in food legislation? This post may be helpful to you, as it highlights the issue of allergens and substances causing intolerances addressed by Regulation 1169/2011 on the provision of food information to consumers (“the Regulation”). You may recall that the rules laid down in the Regulation apply to food business operators at all stages in the food chain, where their activities concern the provision of food information to consumers. The new rules will in principle enter info force on 13 December 2014. However, in order to avoid time constraints by the end of 2014, a large number companies aim acting in compliance as soon as possible. What should be taken into account?
Your company wants to provide bullet proof information on the food products it offers for sale in view of the upcoming changes in food legislation? This post may be helpful to you, as it highlights the issue of allergens and substances causing intolerances addressed by Regulation 1169/2011 on the provision of food information to consumers (“the Regulation”). You may recall that the rules laid down in the Regulation apply to food business operators at all stages in the food chain, where their activities concern the provision of food information to consumers. The new rules will in principle enter info force on 13 December 2014. However, in order to avoid time constraints by the end of 2014, a large number companies aim acting in compliance as soon as possible. What should be taken into account?
The issue of allergergies/intolerances
As allergies and/or intolerances seem to occur more frequently nowadays, the Regulation aims to ensure that full information is provided to allow the consumer to make an informed choice. The EU here clearly follows the example of the US, where FDA requirements on labeling allergens information already is in place. The Regulation stipulates to this respect that any ingredient or processing aid causing allergies or intolerances used in the manufacture or preparation of the food and still present in the finished product shall be indicated in the list of ingredients.
How to list allergens/substances causing intolerances?
This should be done in descending order or weight, as recorded at the time of their use in the manufacture of the food, where the allergens should be emphasised by using a typeset that clearly distinguishes it from the rest of the ingredients, by means of font, style or background colour. If the product does not contain a list of ingredients, then it should mention as a minimum: ”contains….” followed by the allergens. For the time being, 14 types of allergens have been distinguished, but the Commission is at liberty to extend the current list on the basis of a delegated act.
Allergens obligations also for non-prepacked food
Whereas EU food legislation used to apply only to prepacked food, it is striking to note that the obligation to provide information on allergens/substances causing intolerances also applies to non-prepacked food. More precisely, such obligation exists for foods that are offered for sale to the final consumer or to mass caterers without prepackaging, or where foods are packed on the sales premises at the consumer’s request or prepacked for direct sale. So do not be surprised to find a warning at the cheese corner of your Albert Heyn grocery stating that the products sold contain lactose, beind one of the allergens defined under the Regulation.
Stricter national requirements/means of providing information
More particulars, such as the quantity of the allergens or its country of origin, may need to be mentioned too, but only if such has been declared mandatory under national law. The sensitive area of allergens allows Member States to formulate stricter requirements as well (albeit within the Regulation’s framework). However, they are also at liberty to adopt national measures concerning the means through which the information on allergens are to be made available.
How to handle non prepacked food requirements?
It can only be hoped for the Member States adopting national measures on the means of providing information in this area, adopt a practical approach. Although providing information “upon request” is not to be considered as a “means of providing information”, national measures may stipulate that detailed allergen/intolerance information may be given upon request of a consumer. However, such information should be provided in a conspicuous place and in such a way that it is easily visible and clearly legible. This is a practice that restaurants and cafes will need to get used to.
Conclusions
Allergies and substances causing intolerances are in the centre of interest. Many companies whom previously did not have to deal with information on allergies will find that the Regulation also applies to their business. In addition to getting acquinted with and stay posted on the EU allergens requirements, it is important to keep an eye on national legislation in this area. Member States are at liberty to formulate stricter national requirements here. Therefore, despite the aim of harmonization that the Regulation aims to achieve, obtaining local advice for the launch of a new product continues to be a sound plan. Also, stay tune to FoodHealthLegal, as we will provide you with information on requirements for the name of your product and other labeling requirements shortly.
Food information all over the place
Posted: August 14, 2013 Filed under: Food, Information, Nutrition claims Leave a comment »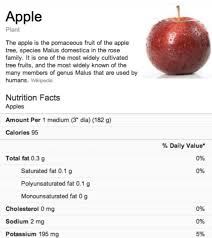 Food information services by Google
Food information services by Google
Recently, Google added nutritional information into its search functionality relating to more than 1,000 food items ranking from fruits to vegetables and from meats to complete meals. The new functionality is a part of Google’s Knowledge Graph that was launched in May 2012 in the US. Knowledge Graph is a database interconnecting various search results in order to enhance understanding. By offering information in this way, Google aims developing its information engine into a knowledge engine. As to food information, Google wants to help its users to make healthier choices – so it says. For examples how Google’s food information service will be operated, reference is made to its blogspot.
Food information in the EU
In the European Union, food information is considered to be of the essence as well. To that end, rules on food information were revamped under Regulation 1169/2011 (“the Regulation”) on the provision of food information to consumers . This Regulation will enter into force on 13 December 2014 and will bring about a great number of changes in the food information landscape in Europe. On the one hand, the Regulation aims to ensure a high level of protection of consumers’ health and interests by enabling them in making informed choices. On the other hand, the Regulation aims to realise free movement of legally produced and marketed food. But how is this going to be achieved?
Fair information practices
Firstly, food information shall not be misleading (a) as to the characteristics of the food, (b) by attributing to the food effects or properties it does not possess, (c) by suggesting that the food possesses special characteristics when in fact all similar food possess such characteristics, (d) by suggesting the presence of a particular food or an ingredient, while in fact substitution with another food or ingredient occurred. It should be stressed that food information comprises all information about a food made available to a final consumer. So not only product labels, but also information published on company website, leaflets, advertisements etc.
Specific guidelines for mandatory information
Secondly, the Food Information Regulation provides specific guidelines for mandatory food information, such as the name of the food, the list of ingredients etc. These guidelines relate for instance to the legibility of this information, requiring a minimum font size of 1,2 mm for the x-height, defined in Annex IV to the Regulation. They also relate to the place where mandatory information is shown, requiring that such information appears directly on the package or on a label attached thereto, so not on a separate leaflet. In cases of distance selling, most of the mandatory information should be available before the purchase is concluded. i.e. it should clearly appear on the website where the food at stake is offered for sale. Furthermore, these guidelines cover language requirements, stipulating that mandatory information shall appear in a language easily understood by the consumers of the Member States where a food is marketed. For the Netherlands, this would clearly be Dutch, but the Member States also have the authority to stipulate that the particulars shall be given in one or more languages of the official languages of the European Union. Such language could be used as an alternative but also in addition to the national language.
Nutrition declarations
Thirdly, nutrition declarations have become mandatory under the Regulation. Such declarations shall imperatively include the following seven elements contained in the food product: (1) energy value, (2) amounts of fat, (3) amounts of saturates, (4) amounts of carbohydrates, (5) amount of sugar, (6) amount of proteins and (7) amounts of salt. Furthermore, they may be supplemented with another six elements such as starch and fibre. The nutrition declaration should come in the form of a table, if space permits, and may be placed on the side or on the back of a packaging (not necessarily at the front side). The information provided must be given per 100 mg or 100 ml – only in addition thereto, information per portion can be given, provided that the portion is quantified. In order to indicate reference intakes of food ingredients, quite often the term GDA (Guideline Daily Amount) is used. This is not a requirement under the Regulation, but if used, this should be done in a way consistent with the Regulation. For vitamins and minerals, it is mandatory however to use the term RDA (Recommended Daily Amount).
Conclusion
The Food Information Regulation brings about such important modifications for food information, that it is very likely that the packaging of most of the products marketed in Europe needs to be adjusted. The nutrition declarations that Google intends to publish for various foods are just one element of those modifications. It is thereby ironic that in an attempt to harmonize the rules on food information, the Food Information Regulation in the same time creates barriers to EU-wide trade, by means of the language requirements for food information. Furthermore, even if the Regulation regulates food information in great detail (e.g. font prescribed font size), it is questionable whether that framework will work for B2B transactions or in cases where the food is not sold to final consumers (e.g. mass caterers). This is certainly food for further thought. Stay tuned to FoodHealthLegal!